Medtronic plc (NYSE:MDT) has made another encouraging move with respect to its Aortic & Peripheral Vascular (APV) business. The company recently announced the receipt of regulatory approval for IN.PACT Admiral Drug-Coated Balloon (DCB) from the Japanese Ministry of Health, Labour and Welfare (“MHLW”).
Medtronic’s IN.PACT Admiral DCB gained approval for treating peripheral artery disease (PAD) in the upper leg, - particularly thigh (superficial femoral arteries and behind the knee (popliteal arteries). However, before the commercial launch of the product, Medtronic has to collaborate with Japanese MHLW to attain reimbursement approval. The company believes that reimbursement approval will widen the global customer base for IN.PACT Admiral DCB.
The Japanese regulatory approval was granted to IN.PACT Admiral DCB on the grounds of successful results from the IN.PACT SFA Japan Trial. This development is in line with the company’s strategy to focus on research and development of high-end medical device products within its core business lines.
We note that the IN.PACT Admiral DCB received FDA approval in December 2014 to treat superficial femoral and popliteal arteries. Moreover, it is commercially available in Europe since its receipt of the CE mark approval in 2009. So far, 200,000 PAD patients in Europe have been treated with the device.
Interestingly, Medtronic’s revenues from the APV division improved 4% year over year (up 5% at constant exchange rate) in first-quarter fiscal 2018. Moreover, Peripheral Vascular business grew low double digits in both atherectomy and drug-coated balloons. The latest development should boost the company’s performance in this segment.
Medtronic’s strategy to gain traction in the peripheral vascular sub-segment seems to be aligned with data provided by MarketsAndMarkets. Per the report, the interventional cardiology & peripheral vascular devices market is expected to see a CAGR of 7.1% from 2016 to 2021 to reach a value of $31.47 billion.
We believe the high incidence of peripheral artery disease due to unhealthy lifestyle and aging population, penetration by the companies in the untapped markets, rising needs for minimally-invasive angioplasty procedures, technological advancements and increasing awareness among people will continue to drive the global acceptance of this technology. In view of these encouraging factors, we believe that the company’s Japanese development for IN.PACT Admiral DCB is strategic and will broaden its customer base.
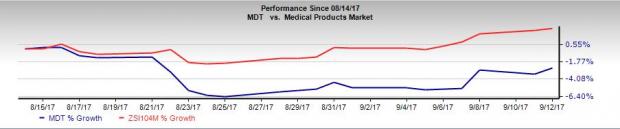
However, over the last month, Medtronic has been underperforming the broader industry. The stock has declined 2.6% in contrast to the industry’s 2.5% gain. It also underperformed the 0.8% gain of the S&P 500 over the same time frame. Nevertheless, we believe the latest Japanese regulatory approval will boost investor confidence in the stock.
Zacks Rank & Stocks to Consider
Medtronic currently carries a Zacks Rank #4 (Sell). A few better-ranked medical stocks in the medical sector are Edwards Lifesciences Corporation (NYSE:EW) , Lantheus Holdings, Inc. (NASDAQ:LNTH) and Amedisys, Inc. (NASDAQ:AMED) . While Edwards Lifesciences sports a Zacks Rank #1 (Strong Buy), Lantheus Holdings and Amedisys carry a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Edwards Lifesciences has a long-term expected earnings growth rate of 15.2%. The stock has rallied roughly 22.2% over the last six months.
Lantheus Holdings has a long-term expected earnings growth rate of 12.5%. The stock has gained 41.2% over the last six months.
Amedisys has a long-term expected earnings growth rate of 18.2%. The stock has gained around 5.3% over the last six months.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Medtronic PLC (MDT): Free Stock Analysis Report
Edwards Lifesciences Corporation (EW): Free Stock Analysis Report
Lantheus Holdings, Inc. (LNTH): Free Stock Analysis Report
Amedisys Inc (AMED): Free Stock Analysis Report
Original post
Zacks Investment Research
