Vertex Pharmaceuticals (NASDAQ:VRTX)
Vertex Pharmaceuticals Incorporated, a global biotechnology company yesterday announced positive results from its Phase 1 and 2 data for their cystic fibrosis treatment.
The studies data showed a 12% point improvement in lung functions of patients with cystic fibrosis who used the triple combination treatment of VX-52, VX-40 AND VX-659.
Vertex Pharmaceuticals Incorporated CMO’s Comments
“These safety and efficacy data are clear and compelling, indicating significant potential benefit for people with CF from each of these three different triple combination regimens,” said Jeffrey Chodakewitz, M.D., Executive Vice President and Chief Medical Officer at Vertex. “We will be collecting and evaluating additional data from these and other studies and will make a decision on which regimen(s) to take forward into pivotal program(s), which we expect to begin in the first half of 2018.”
VRTX Technical Analysis
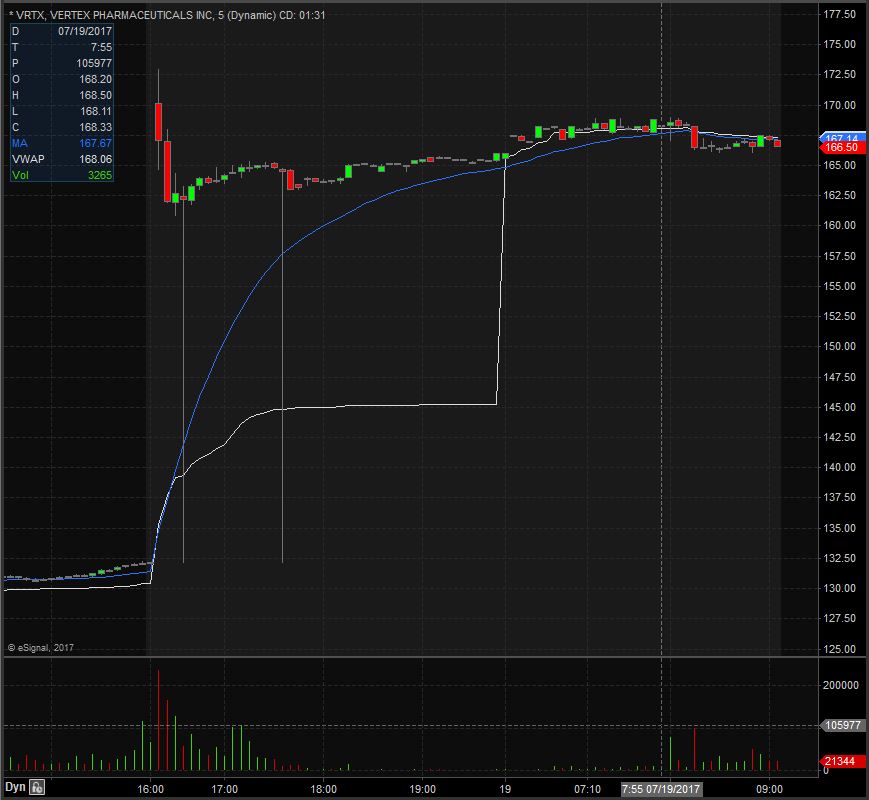
VRTX opened trading yesterday at $129.21 which was exactly the same as the previous day’s trading close. VRTX closed trading yesterday at $132.16 and spiked up after market to $165.45, equivalent to a 25% increase from the closing price. Taking a look at the daily chart we can see that we are in unchartered territory as with the spike up VRTX is now trading at all time highs.
Taking a closer look at the daily chart we can see that before the spike up VRTX had already been in an overall upward trend dating back to March 28th when it traded at $89.67. VRTX has a float of 247.98 million shares and traded 1.90 times the normal daily trading volume on Tuesday.
For trading purposes, I would like to see VRTX open trading on Wednesday above $155.00 and if it does I would be looking to take a long position at the bell. My stop loss would be $2.00 from my entry position fearing anything more than that and the stock would start to fill in the gap up.
Company Profile
Vertex Pharmaceuticals Incorporated discovers, develops, manufactures, and commercializes medicines for serious diseases. The company focuses on developing and commercializing therapies for the treatment of cystic fibrosis (CF) and advancing its research and development programs.
It markets ORKAMBI (lumacaftor in combination with ivacaftor) for the treatment of patients with CF 12 years of age and older who have two copies (homozygous) of the F508del mutation in their cystic fibrosis transmembrane conductance regulator (CFTR) gene; and KALYDECO (ivacaftor) for the treatment of patients with CF 2 years of age and older who have the G551D mutation or other specified mutations in their CFTR gene.
The company also develops Tezacaftor (VX-661), a corrector compound that is in a Phase III development program in combination with ivacaftor in multiple CF patients; VX-152 and VX-440 that are CFTR corrector compounds in Phase II clinical trials, as well as VX-659 and VX-445 that are CFTR corrector compounds in Phase I clinical trials; and VX-371, an investigational epithelial sodium channel, which is in a Phase II development program.
In addition, it engages in the research and mid-and early-stage development programs in the areas of oncology, pain, and neurology. The company sells its products primarily to specialty pharmacy providers and wholesalers in North America, as well as government-owned and supported customers internationally.
Vertex Pharmaceuticals Incorporated has collaborations with Cystic Fibrosis Foundation Therapeutics Incorporated; Parion Sciences, Inc.; Crispr Therapeutics AG (NASDAQ:CRSP); Moderna Therapeutics, Inc.; BioAxone Biosciences, Inc.; Merck (NYSE:MRK); and Janssen Pharmaceuticals, Inc. The company was founded in 1989 and is headquartered in Boston, Massachusetts.
