Ultragenyx Pharmaceutical Inc. (NASDAQ:RARE) announced positive data from two phase II studies, evaluating pipeline candidate burosumab (KRN23) in children with X-linked hypophosphatemia (XLH) and adult with tumor-induced osteomalacia (TIO). Data from the studies were presented at the American Society for Bone and Mineral Research (ASBMR).
Notably, in August, Ultragenyx announced filing of a biologics license application (BLA) to the FDA for burosumab for treatment of XLH. The FDA informed that a decision on acceptance of the same will be made within 60 days.
In January, European Medicines Agency (EMA) had accepted the company’s marketing application for burosumab for review. The company now expects an opinion from the EMA’s Committee for Medicinal Products for Human Use (CHMP) later in 2017.
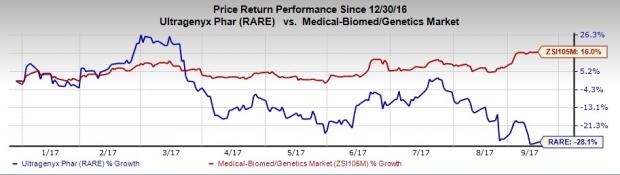
Shares of the company have inched up more than 1% following this news. However, Ultragenyx’s share price movement shows that the stock has significantly underperformed the industry so far this year. Specifically, the stock has plunged 28.1% as against the industry’s 16% gain.
The 64-week pediatric phase II study was designed to assess the safety, pharmacodynamics and efficacy of burosumab, administered every two weeks. The study was conducted on 13 children with the given indication, having completed 40 weeks of treatment.
Data from the study demonstrated better results in serum phosphorus levels, rickets, growth rates and other functional outcomes with use of burosumab, sustained through a prolonged treatment period. The study showed 77% of children achieved normal serum phosphorus levels at week 40.
On the other hand, the 48-week phase II study on adult patients with TIO was also designed to assess the safety and efficacy of burosumab. The trial was conducted on a total of 17 patients. The co-primary endpoints in this study are change in serum phosphorus and key biopsy parameters of osteomalacia. Data from the study confirmed improvement in mean serum phosphorus, renal phosphate reabsorption and serum 1,25 dihydroxy vitamin D levels over 24 weeks’ treatment of 16 patients. However, adverse events were noticed in 44% of patients.
It is important to note that Ultragenyx is conducting the studies under a collaboration and license agreement with Kyowa Hakko Kirin, a Japanese company, to develop and commercialize burosumab.
We remind investors that in June 2016, the FDA had granted a therapy designation to burosumab for treatment of XLH in pediatric patients.
Ultragenyx is making a significant progress with other pipeline candidates as well. Another candidate rhGUS (UX003) is under review in both the United States and the EU for treatment of mucopolysaccharidosis 7 (MPS 7). The company expects a response from the FDA by this year-end and the EU’s feedback is awaited in the first half of 2018.
In the near term, we expect investors’ focus to revolve around the opinion of Committee for Medicinal Products for Human Use (CHMP) on burosumab and for regulatory responses to rhGUS.
Zacks Rank & Stocks to Consider
Ultragenyx currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the pharma sector are Akebia Therapeutics, Inc. (NASDAQ:AKBA) , Aduro Biotech, Inc. (NASDAQ:ADRO) and ACADIA Pharmaceuticals Inc. (NASDAQ:ACAD) , all three carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Akebia’s loss per share estimates narrowed from $4.14 to $3.85 for 2017 and from $1.98 to $1.88 for 2018 over the last 60 days. Its share price soared 65% so far this year.
Aduro Biotech’s loss per share estimates reduced from $1.46 to $1.32 for 2017 and from $1.55 to $1.24 for 2018 over the last 60 days. The company delivered positive surprises in two of the trailing four quarters with an average beat of 2.53%.
ACADIA’s loss per share estimates narrowed from $2.82 to $2.57 for 2017 and from $2.07 to $1.90 for 2018 over the last 60 days. The company came up with positive earnings surprises in two of the last four quarters with an average beat of 7.97%.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Aduro Biotech, Inc. (ADRO): Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD): Free Stock Analysis Report
Ultragenyx Pharmaceutical Inc. (RARE): Free Stock Analysis Report
Akebia Therapeutics, Inc. (AKBA): Free Stock Analysis Report
Original post