Thermo Fisher Scientific Inc. (NYSE:TMO) recently attained emergency use authorization (EUA) from the FDA for its diagnostic test to be used immediately by CLIA high-complexity laboratories in the United States. The test has been developed for the detection of nucleic acid exclusively from SARS-CoV-2, the virus that is responsible for COVID-19.
The emergency authorization of this test has made the company a key player in coronavirus diagnosis.
More About the Test
The authorized test utilizes the Applied Biosystems TaqPath Assay technology and has been developed to deliver patient outcomes within four hours of a sample reaching a laboratory. The estimated time-to-result constitutes of time for sample preparation and instrument analysis.
The EUA test is optimized for use on the company's Applied Biosystems 7500 Fast Dx Real-time PCR instrument. The instrument is covered under the EUA and is already utilized in clinical laboratories all over the world.
This test cannot be used for any other viruses or pathogens.
While the test has not attained FDA approval yet, it has only been authorized for the duration of circumstances justifying the authorization of emergency use of in-vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1). The authorization can be terminated sooner.
Other Companies Working on Coronavirus Testing
Bio-Rad (NYSE:BIO) , through its Exact Diagnostics portfolio, has recently launched a SARS CoV-2 Standard to enable laboratory assay validation of COVID-19 testing.
Quest Diagnostics (NYSE:DGX) recently announced that it is set to launch a COVID-19 test service. This test would facilitate the potential detection of nucleic acid in respiratory specimens of patients who fulfill CDC's clinical criteria for COVID-19 testing. However, the FDA’s review under EUA is still pending.
LabCorp (NYSE:LH) also recently launched its 2019 Novel coronavirus (COVID-19), NAA test, for doctors and other authorized healthcare providers in the United States. The test diagnoses the presence of the underlying virus that causes COVID-19 and should benefit patients who meet current guidelines for evaluation of the disease.
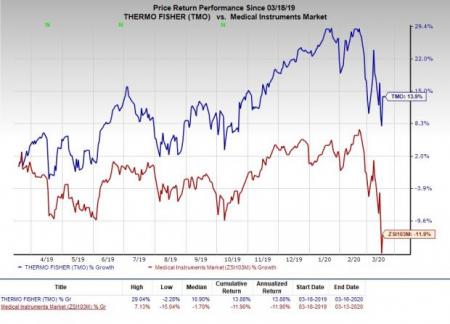
Price Performance
In the past year, the company’s shares have outperformed the industry. The stock has rallied 13.9% against the industry’s 11.9% decline.
Zacks Rank and Key Picks
Thermo Fisher currently has a Zacks Rank # 3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2019, while the S&P 500 gained and impressive +53.6%, five of our strategies returned +65.8%, +97.1%, +118.0%, +175.7% and even +186.7%. This outperformance has not just been a recent phenomenon. From 2000 – 2019, while the S&P averaged +6.0% per year, our top strategies averaged up to +54.7% per year.
See their latest picks free >>
Quest Diagnostics Incorporated (DGX): Free Stock Analysis Report
Laboratory Corporation of America Holdings (LH): Free Stock Analysis Report
Thermo Fisher Scientific Inc. (TMO): Free Stock Analysis Report
Bio-Rad Laboratories, Inc. (BIO): Free Stock Analysis Report
Original post
Zacks Investment Research
