Roche Holding (SIX:ROG) AG’s (OTC:RHHBY) member, Genentech announced that the FDA has accepted the supplemental new drug application (sNDA) for Xofluza (baloxavir marboxil) for the treatment of influenza in people at high risk of complications. The FDA is expected to make a decision on approval by Nov 4, 2019.
The sNDA was supported by results from the phase III CAPSTONE-2 study evaluating a single dose of Xofluza compared with placebo or Tamiflu,(oseltamivir) 75 mg, twice daily for five days, in people aged 12 years or older who are at high risk of complications from the flu. In the study, baloxavir marboxil met the primary and secondary endpoints compared to placebo. Baloxavir marboxil significantly reduced the duration of flu symptoms by more than one day. It also considerably reduced the duration of fever by nearly a day and the levels of virus in the nose and throat from 24 hours through 120 hours.
Xofluza would be the first antiviral medicine approved specifically for the high-risk population.
Xofluza will be further be evaluated in a phase III development program, including pediatric population, post-exposure prophylaxis and severely ill hospitalized people with influenza. The program will also assess the potential of the candidate to reduce transmission in otherwise healthy people.
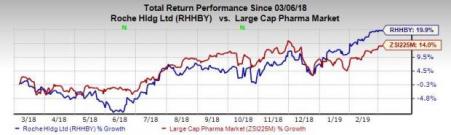
Shares of Roche have increased 19.9% in the past year compared with the industry’s growth of 14%.
We remind investors that in January 2019 the FDA approved the 0.5mL dose of Sanofi’s (NASDAQ:SNY) influenza vaccine, Fluzone Quadrivalent, for treating children falling within the six to 35-month age bracket. The 0.5 mL dosage is already approved for use in patients aged three years or older along with the lesser 0.25 mL dose, which is approved for kids in the six to 35-month age category. Both medicinal measures of the vaccine will be available for the upcoming 2019-2020 flu season.
Notably, in December 2018, the FDA approved Sanofi’s pediatric vaccine, Vaxelis, which is developed for active immunization to prevent six different diseases in minor patients aged six weeks to four years. Vaxelis has been jointly developed by Sanofi (PA:SASY) and Merck (NYSE:MRK) . Both the companies are working on its production, with a commercial launch expected not before 2020.
Also, in January 2018, GlaxoSmithKline plc (NYSE:GSK) received approval from the FDA’s Center for Biologics Evaluation and Research for the label expansion of influenza vaccine — Fluarix Quadrivalent. The vaccine has been approved for infants aged six months or older.
Zacks Rank
Roche currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1% and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
Roche Holding AG (RHHBY): Free Stock Analysis Report
Sanofi (SNY): Free Stock Analysis Report
GlaxoSmithKline plc (GSK): Free Stock Analysis Report
Merck & Co., Inc. (MRK): Free Stock Analysis Report
Original post
Zacks Investment Research