Roche Holding (SIX:ROG) AG’s (OTC:RHHBY) , member, Genentech announced that the FDA has approved a label expansion for Actemra (tocilizumab). Actemra is now approved for the treatment of chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome (CRS) in patients aged between two years and older. CRS, which is caused by an overactive immune response, has been identified as a potentially severe and life-threatening side effect of CAR T cell therapy for certain cancers. This is the first FDA-approved treatment to manage severe cytokine release syndrome associated with CAR T cell therapy.
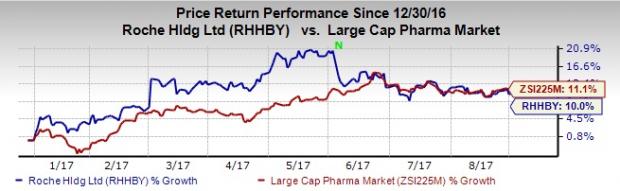
Roche’s shares have underperformed the industry year to date. The stock has gained 10% compared with the industry’s gain of 11.1% in the same time frame.
We note that Actemra is already approved for the treatment of adult patients suffering from moderately to severely active rheumatoid arthritis (RA) who have used one or more disease-modifying antirheumatic drugs (DMARDs), such as methotrexate (MTX) that did not provide enough relief. Actemra is also approved to be used as an IV formulation for patients with active polyarticular juvenile idiopathic arthritis (PJIA) or systemic juvenile idiopathic arthritis (SJIA) aged between two years and older. Actemra subcutaneous injection is also approved for the treatment of adult patients with giant cell arteritis (GCA).
Coming back to the release, the FDA’s approval was based on a retrospective analysis of pooled outcome data from studies of CAR T cell therapies for blood cancers, which evaluated the efficacy of Actemra in the treatment of CRS.
The FDA had granted priority review and orphan drug designation to Actemra for treating CAR T cell-induced CRS based on the rare, severe, and life-threatening nature of CRS and available data on the safety and efficacy of Actemra.
A label expansion of the drug will boost Actemra sales.
On August 30, the CAR-T cells space got a significant boost when the FDA approved Novartis’ (NYSE:NVS) Kymriah (tisagenlecleucel) suspension for intravenous infusion, the first chimeric antigen receptor T cell (CAR-T) therapy, for the treatment of patients up to 25 years of age with B-cell precursor acute lymphoblastic leukemia (ALL) that is refractory or in second or later relapse. Kymriah is the first therapy based on gene transfer approved by the FDA.
Zacks Rank & Key Picks
Roche currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in health care sector include Alexion Pharmaceuticals, Inc. (NASDAQ:ALXN) and Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) . Both Alexion and Regeneron sport a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Alexion Pharmaceuticals’ earnings per share estimates have moved up from $5.32 to $5.61 for 2017 and from $6.53 to $6.92 for 2018 over the last 60 days. The company delivered positive earnings surprises in all the trailing four quarters, with an average beat of 11.12%. The share price of the company has increased 13.2% year to date.
Regeneron’s earnings per share estimates have increased from $12.85 to $14.91 for 2017 and from $15.28 to $16.45 for 2018 over the last 30 days. The company pulled off positive earnings surprises in two of the trailing four quarters, with an average beat of 10.11%. The share price of the company has increased 32.7% year to date.
One Simple Trading Idea
Since 1988, the Zacks system has more than doubled the S&P 500 with an average gain of +25% per year. With compounding, rebalancing, and exclusive of fees, it can turn thousands into millions of dollars. This proven stock-picking system is grounded on a single big idea that can be fortune shaping and life changing. You can apply it to your portfolio starting today. Learn more >>
Roche Holding AG (RHHBY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Alexion Pharmaceuticals, Inc. (ALXN): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Original post
Zacks Investment Research