Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) and partner Sanofi (NYSE:SNY) announced that Dupixent (dupilumab) met its two primary endpoints in a phase III study conducted on patients with uncontrolled, persistent asthma. Data from the LIBERTY ASTHMA QUEST study showed that Dupixent, when added to standard therapies, reduced severe asthma attacks and improved lung function.
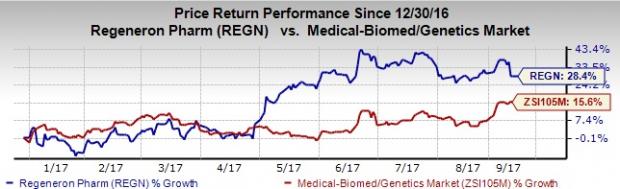
Shares of Regeneron have outperformed the industry year to date. The stock has increased 28.4% as against the industry’s gain of 15.6% in the same time frame.
Dupixent Injection is presently marketed in the United States for the treatment of adults with moderate-to-severe atopic dermatitis (AD). It is the first and only biologic medicine approved for the treatment of adults suffering from AD. It was approved in the United States in March 2017. The European Medicine Agency's Committee for Medicinal Products for Human Use (CHMP) rendered a positive opinion for the marketing authorization of Dupixent, recommending its approval for use in adults with moderate-to-severe atopic dermatitis who are candidates for systemic therapy in Jul 2017.
Coming back to the release, the LIBERTY ASTHMA QUEST study showed that at 52 weeks , in the 300 mg dose group, dupilumab reduced severe asthma attacks by 46% in the overall population. In patients with high levels of eosinophilic cells, dupilumab reduced severe asthma attacks by 60% to 67%.
The study also demonstrated that at 12 weeks, in the same dose group as above, mean improvement in lung function over placebo -- as assessed by forced expiratory volume over one second (FEV1) -- was 9% in the overall population. Lung function improvement was between 210 mL and 240 mL in patients with high levels of eosinophilic cellsBased on this positive data, the companies plan to submit a supplemental Biologics License Application (sBLA) to the FDA by the end of 2017.
Meanwhile, a phase III study of Dupixent in pediatric patients (6-11 years of age) with uncontrolled persistent asthma was initiated in second-quarter 2017. The company expects to initiate two additional studies in the younger atopic dermatitis patients in the second half of 2017 – the first in children between the ages of 6 and 11, and the second in children between the ages of 6months and 5 years. Dupixent is also currently being evaluated for nasal polyps (phase III studies are enrolling) and eosinophilic esophagitis (phase III).
Asthma, a chronic inflammatory disease of breathlessness, and approximately one million U.S. adults and adolescents live with uncontrolled, persistent asthma. Thus it has a significant unmet medical need.
Zacks Rank & Key Picks
Regeneron currently carries a Zacks Rank #1 (Strong Buy). Some top-ranked stocks in health care sector include Alexion Pharmaceuticals, Inc. (NASDAQ:ALXN) and Aduro BioTech, Inc. (NASDAQ:ADRO) . While Alexion sports a Zacks Rank #1 (Strong Buy), Aduro carries Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Alexion Pharmaceuticals’ earnings per share estimates have moved up from $5.32 to $5.61 for 2017 and from $6.53 to $6.92 for 2018 over the last 60 days. The company delivered positive earnings surprises in all the trailing four quarters, with an average beat of 11.12%. The share price of the company has increased 15.4% year to date.
Aduro’s loss estimates per share have narrowed from $1.36 to $1.32 for 2017 and from $1.26 to $1.24 for 2018 over last 30 days. The company came up with positive earnings surprises in two of the trailing four quarters, with an average beat of 2.53%.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market. Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020. Click here for the 6 trades >>
Sanofi (SNY): Free Stock Analysis Report
Alexion Pharmaceuticals, Inc. (ALXN): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Aduro Biotech, Inc. (ADRO): Free Stock Analysis Report
Original post
Zacks Investment Research