Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) along with partner Sanofi (NYSE:SNY) announced that the FDA has granted Breakthrough Therapy status to its experimental candidate, cemiplimab (REGN2810).
Sanofi/Regeneron are looking to get cemiplimab, a checkpoint inhibitor targeting PD-1 (programmed death 1), approved for treating adults with advanced metastatic cutaneous squamous cell carcinoma (CSCC) type of skin cancer. The companies anticipate submitting a biologics license application (BLA) for cemiplimab to the FDA in the first quarter of 2018.
The FDA’s Breakthrough Therapy designation aims to expedite the development and review of drugs, intended to treat serious or life-threatening conditions and provide access to patients as soon as possible.
Significantly, shares of Regeneron have declined almost 6% despite the good news as AbbVie (NYSE:ABBV) announced its skin treatment candidate upadacitinib to having met primary endpoints in phase IIb study which in turn might increase market competition for cemiplimab. However, shares of the company have outperformed the industry year to date. The stock has surged 28.5% compared with the industry’s 15.7% rally during the period.
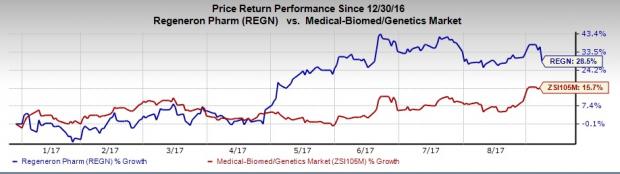
We remind investors that in June, the companies reported positive, preliminary results from two expansion cohorts of phase I study (n=400), evaluating cemiplimab in patients with advanced CSCC. Data from the study showed that treatment with cemiplimab achieved an overall response rate (ORR) of 46.2% and a disease control rate (DCR) of 69.2%. The data was presented at the 2017 American Society of Clinical Oncology Annual meeting.
Another phase II trial, EMPOWER-CSCC 1, is examining REGN2810 in metastatic CSCC and is currently in the enrolment phase. Additionally, a phase III study evaluating REGN2810 as a first-line treatment for non-small cell lung cancer was initiated in the second quarter of 2017.
Notably, cemiplimab is put under a joint development program by Regeneron and Sanofi, post a global collaboration agreement, entered in July 2015. Regeneron had also inked clinical study agreements with other pharmacy biggies namely, Inovio Pharmaceuticals, Inc. (NASDAQ:INO) and SillaJen, Inc. in the second quarter of 2017 to assess cemiplimab in combination with their respective cancer candidates.
Per the company’s press release, CSCC is the second most common and deadliest skin cancer after melanoma in the United States. The disease is also responsible for most deaths among non-melanoma skin cancer patients. Although it is easier to apprehend the condition in early stages, it becomes quite difficult to treat the same once progressed to advanced stages. Hence, the candidate is expected to provide the company with access to a market promising huge potential.
Zacks Rank
Regeneron currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Sanofi (SNY): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Inovio Pharmaceuticals, Inc. (INO): Free Stock Analysis Report
Original post
Zacks Investment Research