Novartis AG (NYSE:NVS) announced that the FDA accepted its generics’ arm Sandoz’s Biologics License Application (BLA) for a proposed biosimilar version of Roche Holding (SIX:ROG) AG’s (OTC:RHHBY) Rituxan (rituximab).
The application was accepted under the 351 (k) pathway.
We note that Rituxan is approved for the treatment of blood cancers including non-Hodgkin's lymphoma (follicular lymphoma and diffuse large B-cell lymphoma) and chronic lymphocytic leukemia, as well as immunological diseases such as rheumatoid arthritis.
We remind investors that Sandoz is a leader in biosimilar market with five marketed biosimilars. The company plans to launch five major oncology and immunology biosimilars between 2017 and 2020. This includes biosimilar rituximab, which was approved by the European Commission in June 2017 (marketed as Rixathon).
In August 2016, Sandoz’s Erelzi, a biosimilar version of Amgen’s (NASDAQ:AMGN) Enbrel gained approval in the United States for five indications of the reference drug. The European Medicines Agency has also accepted the company’s Marketing Authorization Applications for biosimilar versions of Humira (adalimumab) and Remicade (infliximab) for review.
We believe that the approval of new drugs and label expansion of existing drugs will boost the top line, going forward. Sandoz’s performance in the last quarter was disappointing as it grapples pricing pressure.
Earlier, the company announced results from a phase III study of 870 patients with stage III BRAF V600E/K mutation-positive melanoma after complete surgical resection treated with the combination of Tafinlar (dabrafenib) + Mekinist (trametinib). The three-year relapse-free survival rate for patients treated with the combination was 58%, compared to 39% with placebo.
Novartis is currently going through a transitional stage. The company’s blockbuster drug, Diovan, is facing stiff generic competition in the United States, EU and Japan. Gleevec lost exclusivity in the United States in February 2016. The company also lost patent protection for the drug in EU in December 2016 leading to generic competition. The loss of patent protection for these top-selling drugs continue to hurt sales.
Of late, the performance of the Alcon business has been quite disappointing due to lower surgical equipment sales due to competition faced by intraocular lens and a slowdown in demand for equipment purchases. Despite the restructuring initiatives of the company, the segment’s performance remains dismal. In particular, the surgical business is taking longer to turnaround.
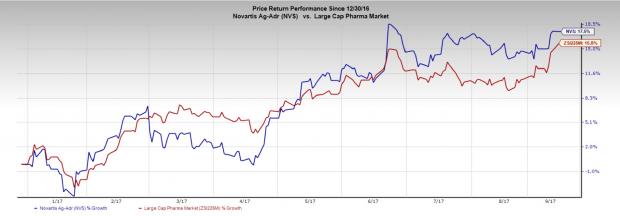
Novartis’ stock has rallied 17.5% in the year so far compared with the industry’s 15.8% gain.
Last week, Novartis announced that its Chief Executive Officer, Joseph Jimenez will step down from his position in 2018. Vasant Narasimhan, M.D., Global Head of Drug Development and Chief Medical Officer, will replace him effective Feb 1, 2018.
Zacks Rank & Key Pick
Novartis currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the healthcare sector is Aduro Biotech, Inc. (NASDAQ:ADRO) which carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Aduro Biotech’s loss per share estimates narrowed from $1.44 to $1.32 for 2017 and from $1.33 to $1.24 for 2018 over the last 30 days. The company delivered positive surprises in two of the trailing four quarters with an average beat of 2.53%.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020. Click here for the 6 trades >>
Roche Holding AG (RHHBY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Aduro Biotech, Inc. (ADRO): Free Stock Analysis Report
Original post
Zacks Investment Research
