Portola Pharmaceuticals, Inc. (NASDAQ:PTLA) recently announced that the FDA has accepted its resubmitted biologics license application (BLA) for the reversal agent for Factor Xa inhibitors, AndexXa (Andexanet Alfa). A decision from the U.S. regulatory body is expected on Feb 2, 2018.
AndexXa has been developed for patients treated with a direct or indirect Factor Xa inhibitor, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding.
Notably, AndexXa is also under review in the EU with marketing application filed in the third quarter of 2016.
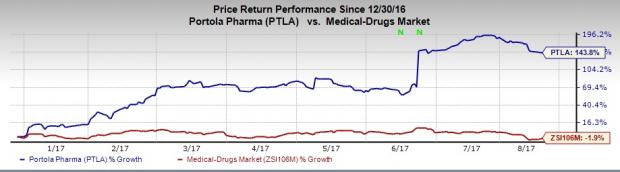
Portola’s shares have significantly outperformed the industry so far this year. The stock has soared 143.8% compared with the broader industry’s decrease of 1.9%.
The resubmission includes additional information requested by the FDA in a complete response letter (CRL) issued to Portola in August last year. In the letter, the FDA had requested for information related to product manufacturing. It had also asked for more data to support the inclusion of direct Factor Xa inhibitor Lixiana (edoxaban) and indirect Factor Xa inhibitor Lovenox (enoxaparin) on the product label.
We remind investors that the BLA was based on positive data from two phase III ANNEXA studies that evaluated the safety and efficacy of AndexXa in reversing the anticoagulant activity of the Factor Xa inhibitors, Xarelto (rivaroxaban) of Johnson and Johnson, Inc. (NYSE:JNJ) and Eliquis (apixaban) of Bristol-Myers Squibb Company (NYSE:BMY) , in older healthy volunteers. Significantly, AndexXa enjoys an Orphan Drug Status in the United States.
Per the company’s press release, more than 90,000 U.S. patients treated with oral Factor Xa inhibitors in 2016 were admitted to hospital due to excessive bleeding. It has also been estimated that more than 150,000 patients taking the injectable Factor Xa inhibitor Lovenox in the United States could benefit from an antidote each year.
With no currently approved antidote for Factor Xa inhibitors yet, there is a high unmet need for the same in the market.
Going ahead, we expect investors’ focus to remain on further details of AndexXa’s regulatory aspect.
Zacks Rank & Stocks to Consider
Portola currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the pharma sector is ACADIA Pharmaceuticals Inc. (NASDAQ:ACAD) , carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
ACADIA’s loss estimates narrowed from $2.82 to $2.65 for 2017 and from $2.07 to $1.98 for 2018 over the last 30 days. The company came up with a positive earnings surprise in two of the trailing four quarters with an average beat of 3.52%.
One Simple Trading Idea
Since 1988, the Zacks system has more than doubled the S&P 500 with an average gain of +25% per year. With compounding, rebalancing, and exclusive of fees, it can turn thousands into millions of dollars.
This proven stock-picking system is grounded on a single big idea that can be fortune shaping and life changing. You can apply it to your portfolio starting today.
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Johnson & Johnson (JNJ): Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD): Free Stock Analysis Report
Portola Pharmaceuticals, Inc. (PTLA): Free Stock Analysis Report
Original post