Exelixis, Inc. (NASDAQ:EXEL) announced that it has completed the submission of a supplemental New Drug Application (sNDA) to the FDA for Cabometyx tablets for patients with previously untreated advanced renal cell carcinoma (RCC).
The sNDA submission was based on positive results from phase II trial, CABOSUN, in patients with previously untreated advanced RCC with intermediate- or poor-risk disease per the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC).
An independent radiology review committee has confirmed the primary efficacy endpoint results of investigator-assessed progression-free survival (PFS) in the CABOSUN trial. The study compared Cabometyx to Pfizer’s (NYSE:PFE) Sutent in the first-line treatment of intermediate- or poor-risk advanced RCC patients. As per the analysis from the committee, Cabometyx demonstrated a clinically meaningful and statistically significant reduction in the rate of disease progression or death as measured by PFS.
In May 2016, Exelixis announced that CABOSUN met its primary endpoint as Cabometyx demonstrated a statistically significant and clinically meaningful improvement in PFS compared to Sutent in patients with advanced intermediate- or poor-risk RCC as determined by investigator assessment.
We remind investors that Cabometyx was approved by the FDA in Apr 2016 for the treatment of patients with advanced RCC who received prior anti-angiogenic therapy. A potential label expansion of the drug will further boost results.
New patient starts, refills for patients already on therapy and continued expansion of the prescriber base for Cabometyx are driving the drug’s sales. The sequential increase in Cabometyx sales was driven by growth in prescriptions.
Exelixis has collaborated with Bristol-Myers Squibb Co. (NYSE:BMY) and Roche Holdings (OTC:RHHBY) for the development of the drug in combination with immunotherapy agents.
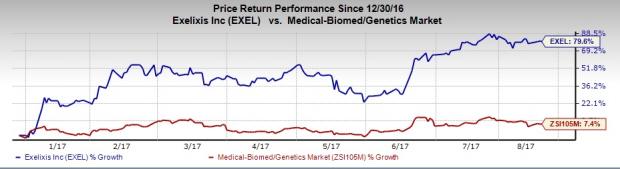
Exelixis’ share price has increased 79.6% year to date compared with the industry’s gain of 7.4%.
Earlier in the week, Bristol-Myers announced disappointing top-line results from the CheckMate-214 trial which investigated immuno-oncology drug Opdivo in combination with Yervoy versus Sutent in intermediate and poor-risk patients previously untreated advanced or metastatic RCC. The trial did not meet its primary endpoint.
We note that renal cell carcinoma (RCC) is the most common type of kidney cancer in adults and accounts for more than 100,000 deaths worldwide each year. Among these, clear-cell RCC is the most prevalent type of RCC and constitutes 80-90% of all cases thereby underlying the demand for the same.
Hence a potential approval of Cabometyx for previously untreated advanced or metastatic RCC will boost sales.
Zacks Rank
Exelixis currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
One Simple Trading Idea
Since 1988, the Zacks system has more than doubled the S&P 500 with an average gain of +25% per year. With compounding, rebalancing and exclusive of fees, it can turn thousands into millions of dollars.
This proven stock-picking system is grounded on a single big idea that can be fortune shaping and life changing. You can apply it to your portfolio starting today. Learn more >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Pfizer, Inc. (PFE): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Original post
Zacks Investment Research
