Murray Hill, NJ-based C. R. Bard, Inc. (NYSE:BCR) recently announced the premarket approval of LUTONIX 035 Drug Coated Balloon PTA Catheter (DCB) by the FDA. The platform has been declared as safe and effective for end-stage renal disease (ESRD) patients with stenotic lesions in dialysis arteriovenous fistulae (AV).
An AV fistula is an abnormal connection or passageway between an artery and a vein, surgically created for hemodialysis treatments. Lutonix 035 is available in the U.S. markets now.
The FDA go-ahead of LUTONIX 035 platform is backed by favorable outcome of a clinical trial, named LUTONIX AV. This is an investigational device exemption (IDE) trial using drug-coated balloons for treating patients with stenotic lesions in AV fistulae.
The Lutonix DCB Platform at a Glance
The Lutonix drug coated balloon (DCB) is a flagship product of the company. It an angioplasty balloon which is coated with a therapeutic dose of the drug paclitaxel, used to treat patients with peripheral arterial disease (PAD). In the recent past, the LUTONIX 035 platform Catheter got a favorable response on the regulatory front for treatment of superficial femoral artery (SFA) and popliteal artery disease.
The early market acceptance of the device has been impressive and the company is likely to witness an expanded adoption of this technology as the market continues to mature. Also, the U.S. Centers for Medicare and Medicaid Services (CMS) approved a pass-through payment for the Lutonix DCB.
We believe the latest regulatory development will ease the stiff pricing environment and boost adoption of the device. In this regard, two major competitors of Bard in the hemodialysis space include Fresenius Medical Care (NYSE:FMS) and DaVita Healthcare Partners, Inc. (NYSE:DVA) .
Favorable Global Trends
More than 2 million patients undergo hemodialysis treatments, with each treatment process lasting for almost four hours and occurring up to thrice a week.
Buoyed by factors like increasing number of ESRD patients, rapid growth in the aging population and preference for dialysis treatments over kidney transplants, the global Hemodialysis and Peritoneal dialysis markets has been growing manifold. A research report by the Markets & Markets shows that the market is expected to reach a worth of $83.89 billion by 2021, at a CAGR of 6.0%.
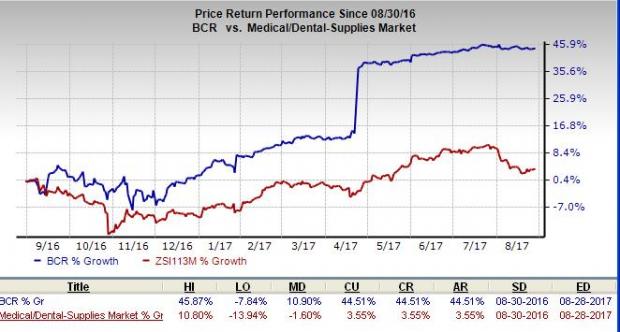
Share Price Moves Up
Over the past one year, Bard has added 44.5%, comparing favorably with the S&P 500’s 11.9% over the same time frame. Furthermore, the current level is way higher than the broader industry’s gain of just 3.6% over the same time frame. Meanwhile, the estimate revision trend for the current quarter was unfavorable with four estimates moving down in the last two months, compared to no movement in the opposite direction. Notably, the current quarter estimates of the stock fell 1.7% to $2.94 over the last two months.
Bard carries a Zacks Rank #3 (Hold).
Key Pick
A better-ranked stock in the broader medical sector is Edwards Lifesciences Corp. (NYSE:EW) . Notably, the company sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Edwards Lifesciences has a long-term expected earnings growth rate of 15.2% and represents an impressive year-to-date return of 21.2%.
Zacks' 10-Minute Stock-Picking Secret
Since 1988, the Zacks system has more than doubled the S&P 500 with an average gain of +25% per year. With compounding, rebalancing, and exclusive of fees, it can turn thousands into millions of dollars.
But here's something even more remarkable: You can master this proven system without going to a single class or seminar. And then you can apply it to your portfolio in as little as 10 minutes a month.
Fresenius Medical Care Corporation (FMS): Free Stock Analysis Report
Edwards Lifesciences Corporation (EW): Free Stock Analysis Report
DaVita HealthCare Partners Inc. (DVA): Free Stock Analysis Report
C.R. Bard, Inc. (BCR): Free Stock Analysis Report
Original post
