This week there were the usual regulatory and pipeline updates from the biotech sector. Juno (NASDAQ:JUNO) , a company focused on immuno-oncology treatments, was in the news with the FDA first placing a clinical hold on a pivotal study and then lifting it. Amgen (NASDAQ:AMGN) also got some positive news flow on the regulatory front with the FDA approving a new dosing option for its PCSK9 inhibitor and its biosimilar version of Humira getting a positive recommendation from an FDA advisory panel.
Recap of the Week’s Most Important Stories
1. It’s been quite a week for immuno-oncology focused Juno which first saw its shares slump on a FDA clinical hold and is now looking at a recovery with the hold being lifted. Last week, shares fell 31.9% when the company’s pivotal phase II study (ROCKET) was placed on clinical hold by the FDA due to two deaths (Read more: Juno Suffers Setback, Pivotal Study on Clinical Hold). But with the FDA lifting the clinical hold yesterday and the company resuming the study under a revised protocol, Juno should recover most of the lost ground (Read more: Comeback for Juno Stock? FDA Lifts Clinical Hold).
2. Amgen also got some good news on the regulatory front – firstly, the company’s biosimilar version of AbbVie’s (NYSE:ABBV) Humira got a positive FDA advisory panel vote and secondly, the company’s monthly single-dose administration option for its PCSK9 inhibitor, Repatha, gained FDA approval. This makes Repatha the first and only PCSK9 inhibitor to offer a monthly single-dose delivery option, thereby giving it an edge over Regeneron and Sanofi’s Praluent (Read more: Amgen's Repatha Gains FDA Approval for New Dosing Option).
Meanwhile, the FDA’s advisory panel voting in favor of Amgen’s Humira biosimilar, ABP 501, is not surprising considering the favorable FDA briefing documents that were released earlier (Read more: Amgen Humira Biosimilar Briefing Documents Look Good). A decision from the FDA regarding the approval status of ABP 501 should be out by Sep 25, 2016.
3. Shortly after gaining FDA approval for its latest hepatitis C virus (HCV) treatment, Epclusa, Gilead (NASDAQ:GILD) gained EU approval as well for the drug (Read more: Gilead's Pan-Genotypic HCV Drug Epclusa Gains EU Approval). Meanwhile, the EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) has completed its review of Gilead’s blood cancer drug, Zydelig. Earlier this year, safety concerns related to the use of the drug had surfaced.
While the PRAC confirmed that Zydelig’s benefits outweigh its risks in the treatment of chronic lymphocytic leukemia (CLL) and follicular lymphoma, the risk of serious infections with Zydelig was also confirmed. The PRAC has provided some recommendations related to the use of Zydelig which will be passed on to the EMA’s Committee for Medicinal Products for Human Use (CHMP) for adoption of the agency’s final position.
4. CytRx’s (NASDAQ:CYTR) shares plunged on initial results from a late-stage study which showed that aldoxorubicin failed to achieve the primary endpoint compared to investigator's choice therapy in patients with relapsed or refractory soft tissue sarcomas (STS). Although the company pointed out that enrollment was interrupted by a clinical hold and a second analysis will take place later, shares were down 59.8% on the news.
5. AbbVie got Rare Pediatric Disease Designation from the FDA for its experimental cancer treatment, ABT-414. The designation is for the treatment of pediatric patients with EGFR-amplified diffuse intrinsic pontine glioma (DIPG), highly aggressive and difficult to treat brain tumors found at the base of the brain (Read more: AbbVie's ABT-414 Gets Rare Pediatric Disease Status in U.S.).
Performance
The NASDAQ Biotechnology Index was up 5.5% over the last five trading days. All major biotech stocks were up with Alexion (NASDAQ:ALXN) gaining 6.5% over the last five trading days. Over the last six months, Amgen was up 7.4% while Alexion lost 24.5% (See the last biotech stock roundup here: Gilead Gets FDA Nod for HCV Drug, Regulus Hit by Clinical Hold).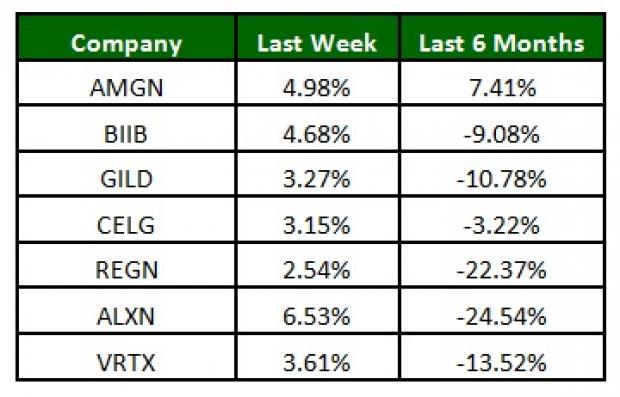
What's Next in the Biotech World?
With second quarter earnings round the corner, watch out for earnings updates as well as the usual pipeline updates and data presentations from biotech companies. Biogen (NASDAQ:BIIB) will be reporting second quarter results next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report >>
ALEXION PHARMA (ALXN): Free Stock Analysis Report
GILEAD SCIENCES (GILD): Free Stock Analysis Report
AMGEN INC (AMGN): Free Stock Analysis Report
BIOGEN INC (BIIB): Free Stock Analysis Report
CYTRX CORP (CYTR): Free Stock Analysis Report
JUNO THERAPEUTC (JUNO): Free Stock Analysis Report
ABBVIE INC (ABBV): Free Stock Analysis Report
Original post
Zacks Investment Research