On Mar 28, we issued an updated research report on AVEO Pharmaceuticals, Inc. (NASDAQ:AVEO) .
AVEO’s Fotivda (tivozanib) is the first approved drug in the company’s portfolio. It was approved in the EU in August 2017 for the first-line treatment of advanced renal cell carcinoma (RCC).
In December 2015, AVEO entered into a license agreement with EUSA Pharma, following which the former granted exclusive right to develop, manufacture and commercialize Fotivda in Europe to EUSA. AVEO receives milestone payments and royalties on Fotivda sales in Europe.
Last November, AVEO announced that it will earn a $2-million milestone fee from EUSA Pharma, triggered by the commercial launch and reimbursement of Fotivda in Germany.
Shares of AVEO have plummeted 56.7% so far this year, against the industry’s increase of 9.6%.
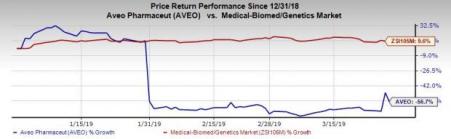
In January, AVEO faced a major setback when it had to delay the submission of a new drug application (NDA) for Fotivda. The company decided not to file an NDA in the United States after the FDA informed that it was not satisfied with the preliminary overall survival (OS) data reported along with the top-line TIVO-3 study data on progression-free-survival (PFS) last November. Shares of the company plunged significantly back then.
Previously, the company intended to submit the NDA in the first half of 2019. However, AVEO has planned a final analysis of OS in August 2019. Data from this interim analysis will be available during the fourth quarter of 2019.
The phase III TIVO-3 study evaluated Fotivda in highly refractory advanced/metastatic RCC patients compared with Bayer (DE:BAYGN) AG’s (OTC:BAYRY) Nexavar (sorafenib). Data from this study along with the previously completed TIVO-1 evaluation will support the filing of a regulatory application for Fotivda’s approval in the United States.
Notably, last December, AVEO entered into a collaboration deal with AstraZeneca (NYSE:AZN) to evaluate the safety and efficacy of the latter's Imfinzi in combination with Fotivda. The phase I/II study will evaluate the combo for the treatment of first-line hepatocellular carcinoma (HCC). The phase I part of the program is expected to begin this year.
AVEO has other promising candidates in its pipeline, including ficlatuzumab, a hepatocyte growth factor inhibitory antibody, currently being evaluated in phase I/II studies for various oncological indications. AV-203, an ErbB3 (HER3) inhibitor antibody targeting growth differentiating factor-15/GDF15, is being examined in phase I study for treating advanced solid tumors. Both AV-380 and AV-353 are in preclinical development for addressing cachexia and pulmonary arterial hypertension (PAH), respectively.
We believe that RCC market holds great potential, as it is among the 10 most common cancers in men and women. Approval of Fotivda in the United States will be a huge boost for the company. However, AVEO is facing setbacks of late, which will hurt the stock significantly. Moreover, the company is heavily dependent on its partners for the development and commercialization of its pipeline candidates. If any of the partners fails to receive regulatory approvals or terminates a deal, AVEO’s future prospects would be severely hampered.
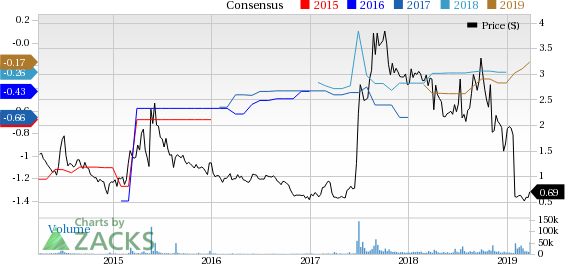
AVEO Pharmaceuticals, Inc. Price and Consensus
Zacks Rank & Another Key Pick
AVEO currently carries a Zacks Rank #2 (Buy). Another top-ranked stock in the same sector is Celgene Corp. (NASDAQ:CELG) , which has a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Celgene’s earnings estimates have been revised 3.5% upward for 2019 and 3.3% for 2020 over the past 60 days. The stock has soared 36.4% in the year so far.
Today's Best Stocks from Zacks
Would you like to see the updated picks from our best market-beating strategies? From 2017 through 2018, while the S&P 500 gained +15.8%, five of our screens returned +38.0%, +61.3%, +61.6%, +68.1%, and +98.3%.
This outperformance has not just been a recent phenomenon. From 2000 – 2018, while the S&P averaged +4.8% per year, our top strategies averaged up to +56.2% per year.
See their latest picks free >>
Bayer Aktiengesellschaft (BAYRY): Free Stock Analysis Report
AstraZeneca PLC (AZN): Free Stock Analysis Report
AVEO Pharmaceuticals, Inc. (AVEO): Free Stock Analysis Report
Celgene Corporation (CELG): Free Stock Analysis Report
Original post
Zacks Investment Research