AbbVie’s (NYSE:ABBV) shares increased almost 2% after the company announced that its oral JAK-1 selective inhibitor, upadacitinib (ABT-494), met primary endpoints in a phase III study for treatment of patients with rheumatoid arthritis (RA).
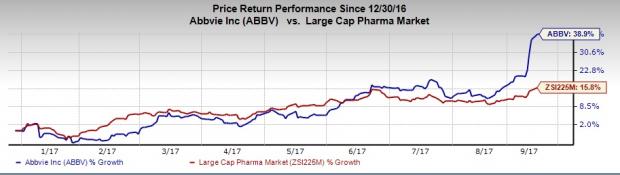
In fact, AbbVie’s shares have rallied 38.9%, outperforming the industry’s 15.8% rise so far this year.
The phase III SELECT-BEYOND study is a multi-center, randomized, double-blind, placebo-controlled parallel group study. It is designed to evaluate the efficacy and safety of two doses (15 mg and 30 mg) of upadacitinib compared with placebo over 12 weeks in patients with moderate-to-severe RA, who are on a stable dose of conventional synthetic DMARDs and have had shown an inadequate response to the dosage.
The study met its primary endpoints with highly statistically significant and clinically meaningful results for both the doses as compared to placebo. The trial’s primary endpoints were ACR20 responses and low disease activity (LDA).
The results showed that 65% of patients, receiving 15 mg and 56% of patients, administered with 30 mg dose once daily of upadacitinib, achieved ACR20 responses after 12 weeks of treatment in comparison to 28% of patients receiving placebo.
LDA was achieved by 43% and 42% of patients in the 15 mg and 30 mg dose arms, respectively, compared with 14% of patients receiving placebo. Additionally, the study met its secondary endpoints too.
Notably, SELECT-BEYOND is the second out of the six ongoing phase III studies in the SELECT RA clinical trial program, conducted on upadacitinib.
Other researches are also underway for upadacitinib for treating Crohn’s disease, ulcerative colitis and atopic dermatitis.
We remind investors that last week, the company announced upadacitinib to having met primary endpoints in a phase IIb study for atopic dermatitis. The company plans to advance the candidate into phase III studies next year for the given indication.
We note that AbbVie already has a strong presence in the RA market with its blockbuster drug, Humira. However, several companies are working on developing biosimilar versions of Humira which could induce competition in the market for the company. Also, the RA market is extremely crowded including drugs like Johnson & Johnson’s Simponi and UCB’s Cimzia among others.
Significantly, Eli Lilly’s (NYSE:LLY) JAK inhibitor, Olumiant, was recently approved in the EU and Pfizer’s (NYSE:PFE) Xeljanz is marketed in the United States for treating RA, which might pose a strong competition for upadacitinib on approval.
Also, Amgen (NASDAQ:AMGN) has already received approval to market a Humira biosimilar but the drug has not been launched yet due to ongoing litigation. If upadacitinib is approved, it may reduce the potential negative impact of Humira generics on AbbVie’s top line.
Zacks Rank
AbbVie currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple (NASDAQ:AAPL) sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 27 billion devices in just 3 years, creating a $1.7 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 6 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2020.
Click here for the 6 trades >>
Pfizer, Inc. (PFE): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
AbbVie Inc. (ABBV): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Original post
Zacks Investment Research