By Gabriella Borter and Carl O'Donnell
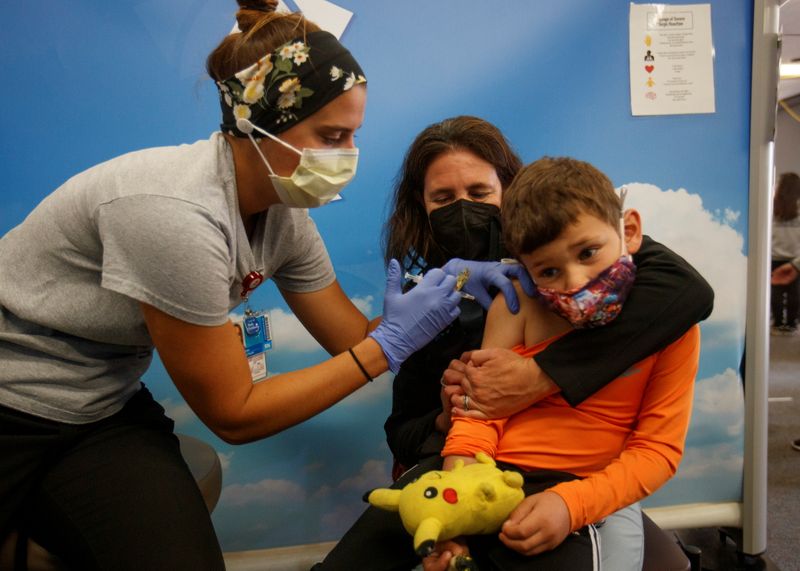
(Reuters) - Seven-year-old Gael Coreas stuck out his left arm fearlessly to receive his first COVID-19 shot at a health clinic in the nation's capital on Wednesday, wincing briefly as cameras flashed to capture the moment.
Coreas was in the first cohort of young children to be inoculated as the United States on Wednesday began administering the COVID-19 vaccine to children ages 5 to 11, the latest group to become eligible for the shots that provide protection against the illness to recipients and those around them.
"We are very excited," Coreas' mother Alma Benavides, a 37-year-old small business owner, said at Mary Center's in Washington. "We waited a long time and it’s very special for me."
On Tuesday, the U.S. Centers for Disease Control and Prevention recommended the Pfizer Inc (NYSE:PFE) https://www.reuters.com/world/us/us-cdc-advisers-vote-covid-19-vaccine-young-children-2021-11-02/BioNTech SE shot for broad use in that age group, four days after it was authorized by the U.S. Food and Drug Administration.
Only a limited amount of the initial 15 million shots being distributed will be available on Wednesday. They are expected to be more widely accessible at pediatrician's offices, children's hospitals and pharmacies next week.
Maria Stout brought her daughters Celeste, 9, and Victoria, 11, to Rady Children's Hospital in San Diego for their first COVID-19 shots on Wednesday morning, after a friend told her the hospital's website had open appointments.
Stout passed along the tip to other parents, some of whom were unable to book appointments, "so it was very fortunate that my friend shared that information with me early," she said.
The big national pharmacy chains, Walgreens Boots Alliance (NASDAQ:WBA), CVS Health (NYSE:CVS) and Rite Aid (NYSE:RAD) are among those offering appointments for this weekend.
"We have had significant demand and scheduling already," CVS Chief Executive Karen Lynch said in an interview on Wednesday. CVS expects to have 2,500 stores administering pediatric shots beginning this weekend.
While about 58% of Americans are fully vaccinated against COVID-19, some 28 million children under 12 have not been eligible until now.
The 10-microgram shot of the Pfizer/BioNTech vaccine authorized for school-age kids - a third the strength given to adolescents and adults - offers protection from the Delta variant of the virus that has led to thousands of pediatric hospitalizations.
'PEOPLE WILL BE MORE COMFORTABLE'
Dr. Monica Gandhi, an infectious disease expert at the University of California, San Francisco, said the approval for this age group was especially important because they are school-aged children.
The vaccine, shown to be more than 90% effective at preventing symptomatic infection in children, provides an avenue for fewer quarantines or school closures and more normal activities and freedoms.
"In elementary schools, it's been really difficult for them to be totally normal without this vaccine because teachers have been worried about transmission," Gandhi said.
The rollout, although too late for these children to be fully protected by Thanksgiving later this month, should make year-end holiday celebrations safer.
"I think people will be more comfortable in the family gatherings not wearing masks because the youngsters now also are vaccinated," said Dr. William Schaffner, an infectious disease expert at Vanderbilt University Medical Center and a non-voting member of CDC's vaccine advisory panel.
Still, it remains unclear how many parents https://www.reuters.com/world/us/some-parents-eager-others-unsure-covid-19-shot-approved-kids-2021-11-03 will jump at the chance. Even many who have been vaccinated themselves are more divided over whether to vaccinate their own younger children, given that severe COVID-19 is much less common for them.
There were no new safety issues in Pfizer's study of the vaccine in thousands of children, but there is also no long-term data for its use.
A few other countries, including China, are already vaccinating children. The European Union and Canadian regulators are currently considering Pfizer's application for the vaccine in this younger age group.
So far, only Pfizer's shot has been authorized for use in the United States for those under age 16.
Moderna (NASDAQ:MRNA) Inc has delayed its request for authorization for its vaccine for children aged 6 to 11 and is waiting on an FDA review of safety data in connection with its application for 12- to 17-year olds.
The states with the highest adult COVID-19 vaccination rates are preparing bigger pushes to get children inoculated than states where hesitancy remains strong, potentially widening the gaps in protection nationwide, public health officials and experts said.
COVID-19 vaccines have emerged as yet another issue exposing deep political divides in the United States that led to opposing stances on vaccinations, face covering and other pandemic restrictions in various parts of the country.