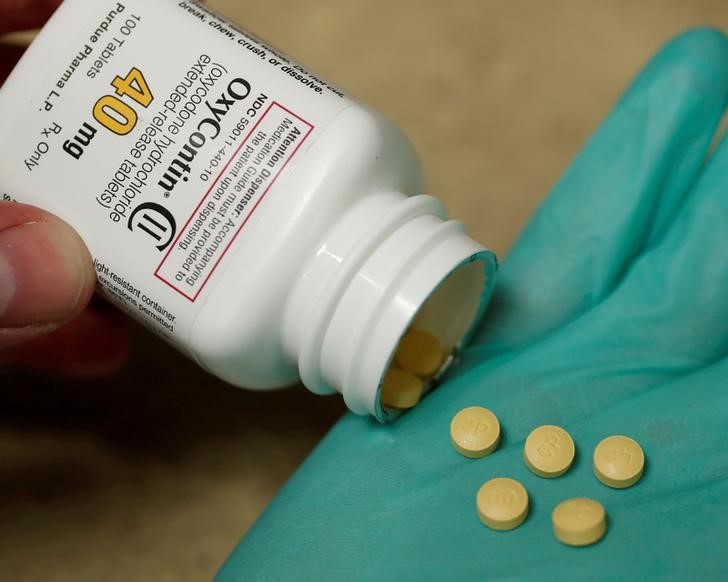
(Reuters) - OxyContin maker Purdue Pharma LP said on Saturday that it has cut its sales force in half and will stop promoting opioids to physicians, following widespread criticism of the ways that drugmakers market addictive painkillers.
The drugmaker said it will inform doctors on Monday that its sales representatives will no longer visit physician offices to discuss its opioid products. It will now have about 200 sales representatives, Purdue said.
"We have restructured and significantly reduced our commercial operation and will no longer be promoting opioids to prescribers," the Stamford, Connecticut-based company said in a statement.
Doctors with opioid-related questions will be directed to its medical affairs department. Its sales representatives will now focus on Symproic, a drug for treating opioid-induced constipation, and other potential non-opioid products, Purdue said.
Opioids were involved in more than 42,000 overdose deaths in 2016, according to the U.S. Centers for Disease Control and Prevention.
Among other opioid producers, Endo International Plc (O:ENDP) agreed in July to pull its Opana ER painkiller after the Food and Drug Administration called for its withdrawal.
Purdue and other drugmakers have been fighting lawsuits by states, counties and cities that have accused them of pushing addictive painkillers through deceptive marketing.
The lawsuits have generally accused Purdue of downplaying OxyContin's addiction risk and of misleading marketing that overstated the benefits of opioids for treating chronic, rather than short-term, pain.
At least 14 states have sued privately held Purdue. Alabama Attorney General Steve Marshall filed a lawsuit on Tuesday accusing Purdue of deceptively marketing prescription opioids.
Purdue is also facing a federal investigation by the U.S. Attorney's Office in Connecticut.
Purdue has denied the allegations in the various lawsuits. It has said its drugs are approved by the U.S. Food and Drug Administration and account for only 2 percent of all opioid prescriptions.
Purdue and three executives pleaded guilty in 2007 to federal charges related to the misbranding of OxyContin and agreed to pay $634.5 million to resolve a U.S. Justice Department probe.
That year, Purdue also reached a $19.5-million settlement with 26 states and the District of Columbia. It agreed in 2015 to pay $24 million to resolve a lawsuit by Kentucky.
U.S. President Donald Trump has drawn criticism for his response to the opioid crisis. He has yet to declare it a national emergency as he pledged to do in August following a recommendation by a presidential commission.