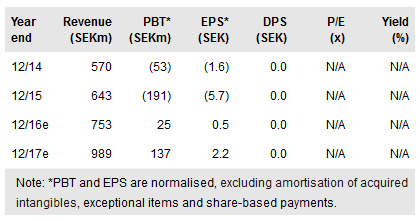
Orexo's (ST:ORX) Q216 results pointed to positive Zubsolv momentum with evidence of net revenue growth, improving margins and encouraging market access developments. Financial discipline contributed to a Q216 SEK12.1m operating profit and a second successive quarter of positive operating cash flow. The new Maryland FFS Medicaid agreement should help boost Zubsolv’s penetration into the public market segment and ongoing expansion in US prescribing rights will be a key growth driver longer term. Ex-US, the recent Mundipharma licensing deal provides access to the global opioid dependence market. In the near term, however, uncertainty due to the ongoing Actavis (NYSE:AGN) litigation weighs on the current share price.
Positive developments for Zubsolv and the US market
The Maryland FFS agreement makes Zubsolv the exclusive preferred product on the Maryland state formulary from July. The HHS increase of the patient cap (to 275 from 100) and Congress passage of the Comprehensive Addiction and Recovery Act 2016 (enactment into law is pending presidential signature) means that legislative changes to increase access to treatment become increasingly tangible.
To read the entire report Please click on the pdf File Below