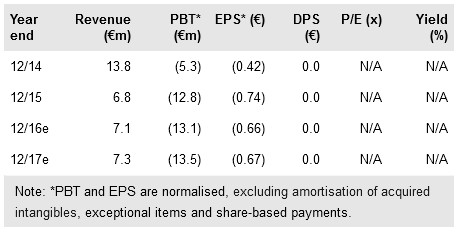
Medigene (DE:MDG1k) is well funded, with a net cash position of €46.8m (FY15). There is a clear strategy in place to advance both its DC vaccine programmes and TCR programme with the funds. We expect newsflow in the near term from its most advanced technology (DC vaccines) in Phase I/II studies for AML and prostate cancer, and the start of the first clinical study with its promising TCR technology. Our rNPV-based valuation remains at €216m.
Advancing DC vaccine programmes
Medigene’s DC vaccine programmes include investigator-initiated trials (IITs) and a company-initiated trial (CIT), both of which are making progress. The CIT is a Phase I/II study in 20 AML patients, started in March 2015. The first six patients have been treated and, following review by the Data and Safety Monitoring Board, Medigene has commenced Phase II with the recent announcement that the first Phase II patient has started treatment. Once patient recruitment is complete, the Phase II trial will take about two years to complete (one year of treatment and one year of follow-up), with expected completion in 2019 and data readout in 2020. The start of Phase II has triggered a €3.2m milestone payment to former Trianta Immunotherapies shareholders. This will be settled by the issue of new shares, already forecast in our model. We also expect data from an IIT in Phase II for use of its DC vaccine in prostate cancer in H116. As outlined previously, the DC vaccine has technical advantages and holds promise as both a mono and combination therapy.
To read the entire report Please click on the pdf File Below