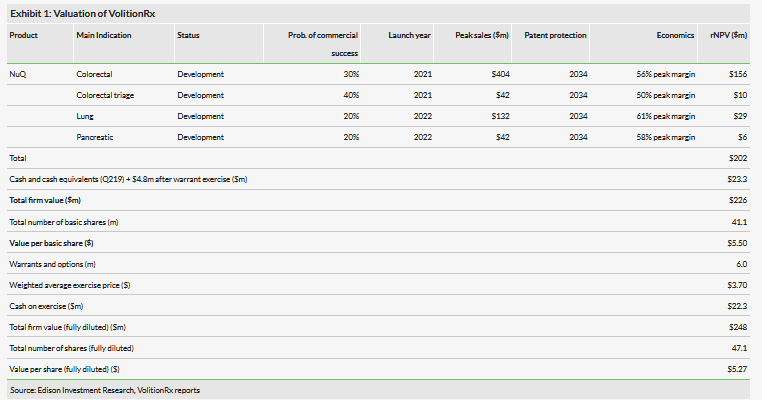
The main highlights of Volitionrx (NYSE:VNRX)’s Q219 results were the establishment of a veterinary subsidiary in Texas, US and the initiation of two lung cancer trials; the first in collaboration with Fosun Long March in China and the second with National Taiwan University. This indicates that lung cancer is gaining traction in R&D and is now the second most advanced indication after colorectal cancer. In July 2019 VolitionRx exercised warrants to raise another $4.8m (ytd it has exercised $16.5m), extending the cash runway to 2021. Our valuation post the warrant exercise is $226m or $5.50/share.
Fosun collaboration and progress in animal health
On 18 July 2019, VolitionRx announced that it had started a clinical trial in China in lung cancer in partnership with Fosun Long March, which is owned by a conglomerate, Shanghai Fosun Pharmaceutical. Fosun Long March is an in-vitro diagnostics company active in the R&D, manufacture and marketing of diagnostic and laboratory instruments and reagents, so it is a suitable partner for Volition. Financial details were not disclosed. VolitionRx already has a presence in Asia via an R&D collaboration with the National Taiwan University and the parties are running a large-scale, prospective study in lung cancer (blood samples from 1,200 subjects). These developments mean that lung cancer has become central to VolitionRx’s product development strategy in addition to colorectal cancer.
New subsidiary established
On 8 August 2019, VolitionRx announced the formation of a subsidiary, Volition Veterinary Diagnostics Development based in Texas, US. A separate subsidiary was deemed as the best corporate structure to explore opportunities in animal health. Although a relatively new area, VolitionRx presented its plans to expand in the veterinary space at the capital markets day in April 2019. Animal health could potentially be a commercially lucrative area given the size of the market, the high unmet need and the lower regulatory hurdle compared with humans.
Valuation: $226m or $5.50/share
Our VolitionRx absolute valuation is somewhat higher at $226m (vs $215m) due to an updated cash position and rolling the model forward, while the valuation per share is virtually unchanged at $5.50 (vs 5.46) due to dilution post the warrant issue. We made no changes to our estimates. Newsflow over the next 12 months includes more proof-of-concept data with the product grade assays, initial results from the Asian clinical trials, progress in colorectal cancer test development and updates on the animal health venture.
VolitionRx is a research client of Edison Investment Research Limited
Business description
VolitionRx is a life sciences company developing novel, simple-to-use, blood-based tests to diagnose a range of cancers and conditions by identifying and measuring nucleosomes in the blood stream. The primary focus is to develop the Nu.Q family of blood-based diagnostics tests for cancer.
R&D update
Nu.Q lung cancer test development accelerates
The main highlights of the Q219 results were the establishment of a subsidiary in Texas, US and the initiation of two lung cancer trials; the first in collaboration with Fosun Long March in China and the second with National Taiwan University. The signing of a memorandum of understanding with Fosun Long March was announced on 28 March 2019. The preliminary plan was to conduct clinical studies in China to investigate several solid cancers. On 18 July 2019, VolitionRx announced that the parties have commenced a clinical trial in China in lung cancer. Financial details have not been disclosed.
As we described in our outlook report in May, VolitionRx already had a presence in Asia via an R&D collaboration with the National Taiwan University. On 7 May 2019, VolitionRx announced the first (at that time) large-scale, prospective study in lung cancer (blood samples from 1,200 subjects). The aim of the study is to develop either a frontline screening test in lung cancer or a triage test after low-dose computed tomography (LDCT) (gold standard currently) to address the low specificity associated with LDCT. The total cost for VolitionRx was estimated at $320k over the next two years (until 2021). The first data readout is expected in Q120.
Historically, VolitionRx’s primary focus has been colorectal cancer, so we presume the interest in lung cancer is largely driven by the Chinese partner. We view this as a strategic opportunity for VolitionRx to access the Chinese market, but also to advance the second cancer indication in its R&D pipeline. To this end, VolitionRx presented the first proof-of-concept data from its own exploratory study in lung cancer using the product-grade assays earlier this year (described in our recent outlook report).
Texas subsidiary to focus on animal health opportunity
On 8 August 2019, VolitionRx announced the formation of a subsidiary, Volition Veterinary Diagnostics Development, based in Texas, US. Animal health expert Nathan Dewsbury has been appointed chief executive officer. A separate subsidiary was deemed to be the best corporate structure for VolitionRx to explore opportunities in animal health, as the company indicated that it would be open for interested partners to take equity stakes in it. The current collaborator, Texas A&M University, has previously expressed an interest in such an investment, but we understand that for now the subsidiary is wholly owned by VolitionRx.
Although a relatively new area, VolitionRx presented its plans to expand in the veterinary space at the capital markets day in April 2019. The feasibility studies showed that using the same assays as in humans, the researchers were able to detect nucleosomes in samples from dogs diagnosed with cancer. Animal health has the potential to be a commercially lucrative area due to the combination of a large market, high unmet need and a substantially lower regulatory hurdle compared with humans. VolitionRx indicated that the first product for dog cancer diagnostics could be ready for market as soon as 2020. Due to the early stage of the product at present, we do not include it in our valuation, but will revisit once more information is available.
Financials and valuation
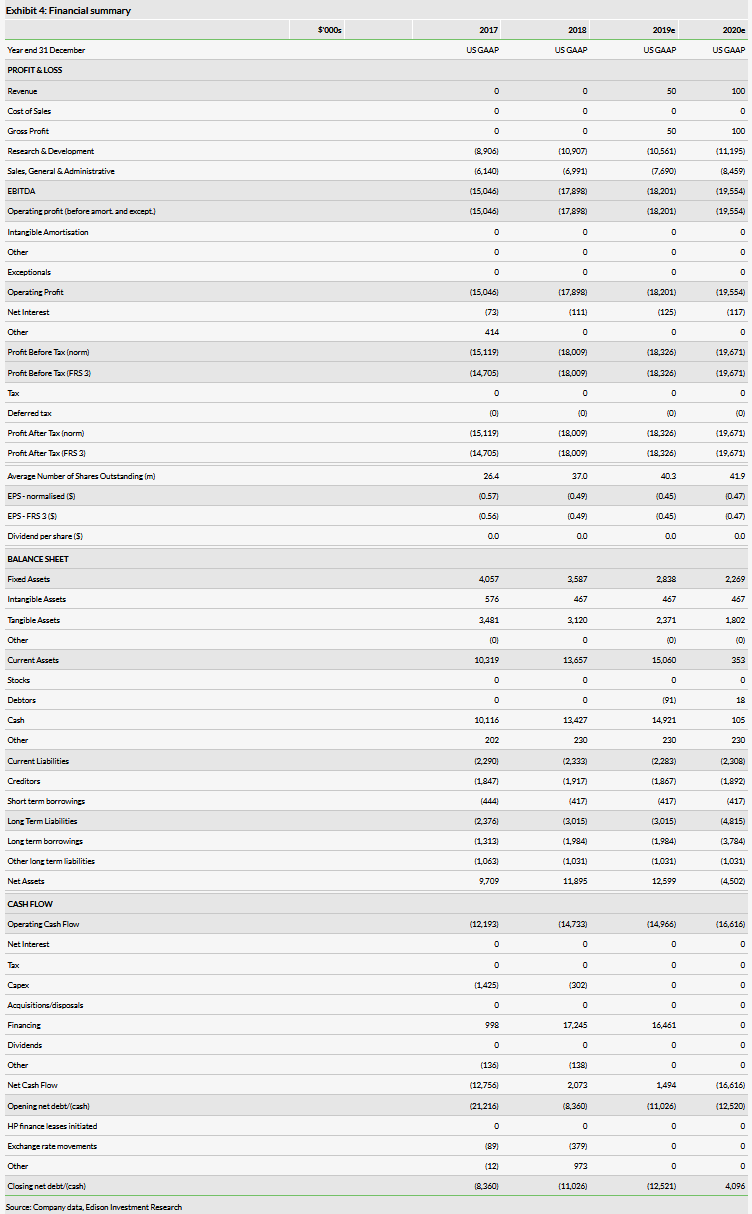
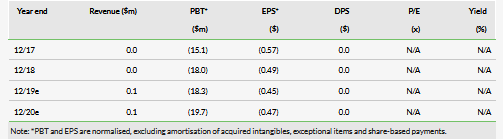
VolitionRx reported no income and an operating loss of $4.1m in Q219, compared to $4.6m a year ago, which is largely in line with our expectations. The company had cash of $18.5m at end-Q219. After the Q219 close, the company exercised a further $4.8m in warrants. In total, VolitionRx has exercised $16.5m in warrants in 2019 showing investor support. VolitionRx had $2.5m in gross debt and $1.0m in lease liabilities at the end of Q219. Assuming a similar level of cash burn, our model now suggests cash runway until the end of 2020 (our model suggests a shortfall of $1.8m, which we add as a long-term debt).
Our VolitionRx absolute valuation is somewhat higher at $226m (previously $215m) due to the updated cash position and rolling our model forward, while the valuation per share is virtually unchanged at $5.50 (vs 5.46) due to dilution post the warrant issue. Our other R&D assumptions, detailed in our previous notes, remain unchanged.
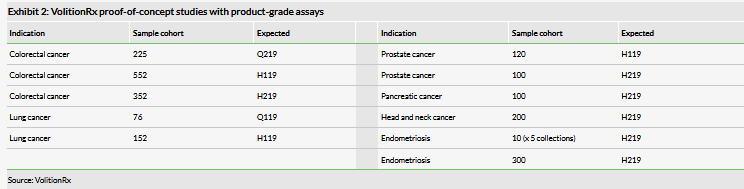
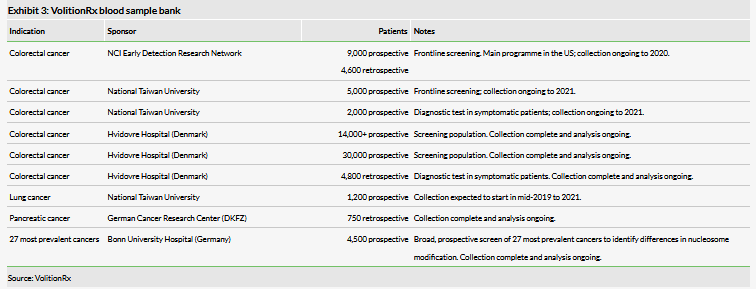
VolitionRx is conducting multiple proof-of-concept studies with the upgraded Nu.Q assays, as described in detail in our last outlook report and summarised on Exhibit 2. The results from those studies, although early, could provide interesting catalysts for the share price. Of the larger programmes (Exhibit 3), VolitionRx has indicated that the interim readout from the lung cancer trial in Asia is expected in Q120. For now we still do not include Nu.Q Vet or Nu.Q Capture commercial potential in our valuation due to early stage of these programmes and lack of details. However, there is potential for the Nu.Q Vet programme to progress relatively quickly compared to cancer diagnostics in humans due to the lower R&D hurdles in animal health.