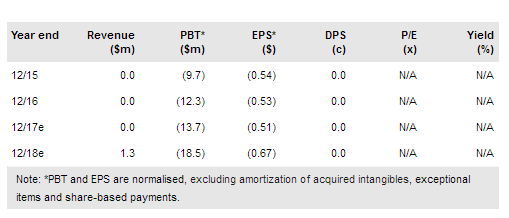
Volitionrx (NYSE:VNRX) will be participating in a 13,500-patient prospective trial investigating its colorectal Nu.Q™ test (and other biomarkers) in the US. The trial will be run in collaboration with the National Cancer Institute (NCI) and the University of Michigan, which will be bearing the majority of its cost. VolitionRx will only pay $3m over the two to three years the trial is expected to take to complete. We are reducing our valuation to $236m (from $272m) or $8.89 per basic share due to the long timeline in the US.
US trial to support PMA filing
For the US trial, 4,677 samples have already been collected and up to an additional 9,000 samples will be prospectively collected from asymptomatic individuals aged 50 years and over. At this scale, this is one of the largest ever trials for a colorectal cancer screening product. After feedback from the FDA, Exact Sciences ran a 10,000-person prospective clinical trial. VolitionRx has stated that it believes its trial should support a PMA, although it has not confirmed this with the FDA.
To read the entire report Please click on the pdf File Below: