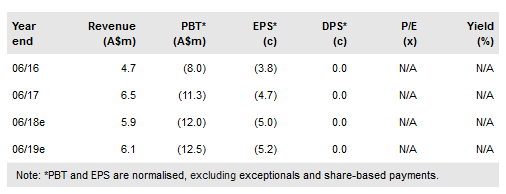
Viralytics Ltd (AX:VLA) continues to report impressive preliminary data for Cavatak in combination with immune checkpoint inhibitors (ICIs), whether administered intravenously (iv) or by intra-tumoural injection. Viralytics is currently recruiting additional patients in expansion cohorts in melanoma, lung and bladder cancers in order to obtain more robust estimates of tumour response rates. It has announced plans to initiate four Phase I studies in additional indications and has commenced planning for a potential pivotal study of Cavatak plus Yervoy in melanoma patients who had failed prior single-agent, anti-PD1 ICI therapy, a serious unmet medical need. Updates on MITCI, CAPRA and Keynote 200 are expected in Q218. We increase our valuation to A$469m or A$1.95/share (vs A$408m and A$1.70/share).
Cavatak/Yervoy responses where ICI therapy failed
Updated preliminary data presented at SITC in November for melanoma patients treated with Cavatak oncolytic virus in combination with Yervoy in the MITCI study included response rates of 29% in patients who had failed prior single-agent PD1 therapy, which compares to response rates of 11-14% reported for Yervoy alone. The Cavatak/Yervoy combo was very well tolerated. Planning has commenced for a pivotal study in this indication which could start in Q418, if the expanded MITCI study continues to deliver positive data.
To read the entire report Please click on the pdf File Below: