TIME to advance to Phase III
Interim data from the first stage of the Phase II/III TIME trial with Transgene's, (TRNG) TG4010 supports the continuation of the trial into Phase III. The results from the study in non-small cell lung cancer (NSCLC) validate the use of the triple-positive activated lymphocytes (TrPAL) biomarker, even though the primary endpoint for TrPAL usage was missed. There was a clinical meaningful improvement in progression free survival (PFS) in patients with lower TrPAL levels. We now consider it likely that Novartis will exercise its option on TG4010. We increase our valuation to €462m.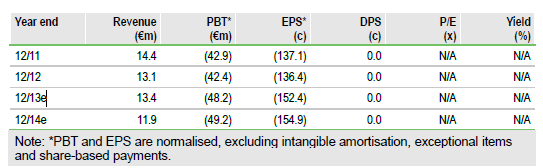
Validation of Transgene’s TrPAL biomarker
The main aim of the first part of the Phase II/III TIME trial with TG4010 in first-line NSCLC in combination with chemotherapy was to confirm the finding of the previous Phase II study that patients with lower levels of TrPAL (activated NK cells) benefited from the therapeutic vaccine TG4010. There was a >25% reduction in risk of progression or death (HR<75%) with TG4010 in the 75% of patients with the lower baseline TrPAL levels, but not in the top quartile (pre-specified analysis).
Trial set to continue into Phase III
The data justify the continuation of the trial into Phase III, however the level of TrPAL used to identify the likely responders will be lowered slightly. The trial missed its primary endpoint; there was no meaningful improvement in PFS caused by TG4010 in patients designated as having TrPAL levels below the “upper limit of normal”, set from the analysis of healthy volunteers in a separate study. It is also possible the next stage of the trial will be restricted to those patients with non-squamous NSCLC (c 55% of lung cancers) not treated with bevacizumab (Avastin), as patients in this patient group had a larger improvement in PFS.
Novartis could exercise option by mid-April
These results could lead to Novartis exercising its option to in-license TG4010 by mid-April, in a deal worth c €700m. If Novartis decides against doing so, based on this data we believe Transgene could find another partner for TG4010.
Valuation: DCF valuation of €462m
We increase our valuation from €390m to €462m, after increasing the likelihood of success for TG4010 by 10% to 60% and of Novartis exercising its option from 60% to 80%. The company has sufficient cash to operate into 2015, so has enough time, if necessary, to find an alternative partner to Novartis.
- English (UK)
- English (India)
- English (Canada)
- English (Australia)
- English (South Africa)
- English (Philippines)
- English (Nigeria)
- Deutsch
- Español (España)
- Español (México)
- Français
- Italiano
- Nederlands
- Português (Portugal)
- Polski
- Português (Brasil)
- Русский
- Türkçe
- العربية
- Ελληνικά
- Svenska
- Suomi
- עברית
- 日本語
- 한국어
- 简体中文
- 繁體中文
- Bahasa Indonesia
- Bahasa Melayu
- ไทย
- Tiếng Việt
- हिंदी
Transgene: Trial Set To Continue Into Phase III
Published 01/09/2014, 09:57 AM
Updated 07/09/2023, 06:31 AM
Transgene: Trial Set To Continue Into Phase III
Latest comments
Loading next article…
Install Our App
Risk Disclosure: Trading in financial instruments and/or cryptocurrencies involves high risks including the risk of losing some, or all, of your investment amount, and may not be suitable for all investors. Prices of cryptocurrencies are extremely volatile and may be affected by external factors such as financial, regulatory or political events. Trading on margin increases the financial risks.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
Before deciding to trade in financial instrument or cryptocurrencies you should be fully informed of the risks and costs associated with trading the financial markets, carefully consider your investment objectives, level of experience, and risk appetite, and seek professional advice where needed.
Fusion Media would like to remind you that the data contained in this website is not necessarily real-time nor accurate. The data and prices on the website are not necessarily provided by any market or exchange, but may be provided by market makers, and so prices may not be accurate and may differ from the actual price at any given market, meaning prices are indicative and not appropriate for trading purposes. Fusion Media and any provider of the data contained in this website will not accept liability for any loss or damage as a result of your trading, or your reliance on the information contained within this website.
It is prohibited to use, store, reproduce, display, modify, transmit or distribute the data contained in this website without the explicit prior written permission of Fusion Media and/or the data provider. All intellectual property rights are reserved by the providers and/or the exchange providing the data contained in this website.
Fusion Media may be compensated by the advertisers that appear on the website, based on your interaction with the advertisements or advertisers.
© 2007-2025 - Fusion Media Limited. All Rights Reserved.