TESARO Inc. (NASDAQ:TSRO)
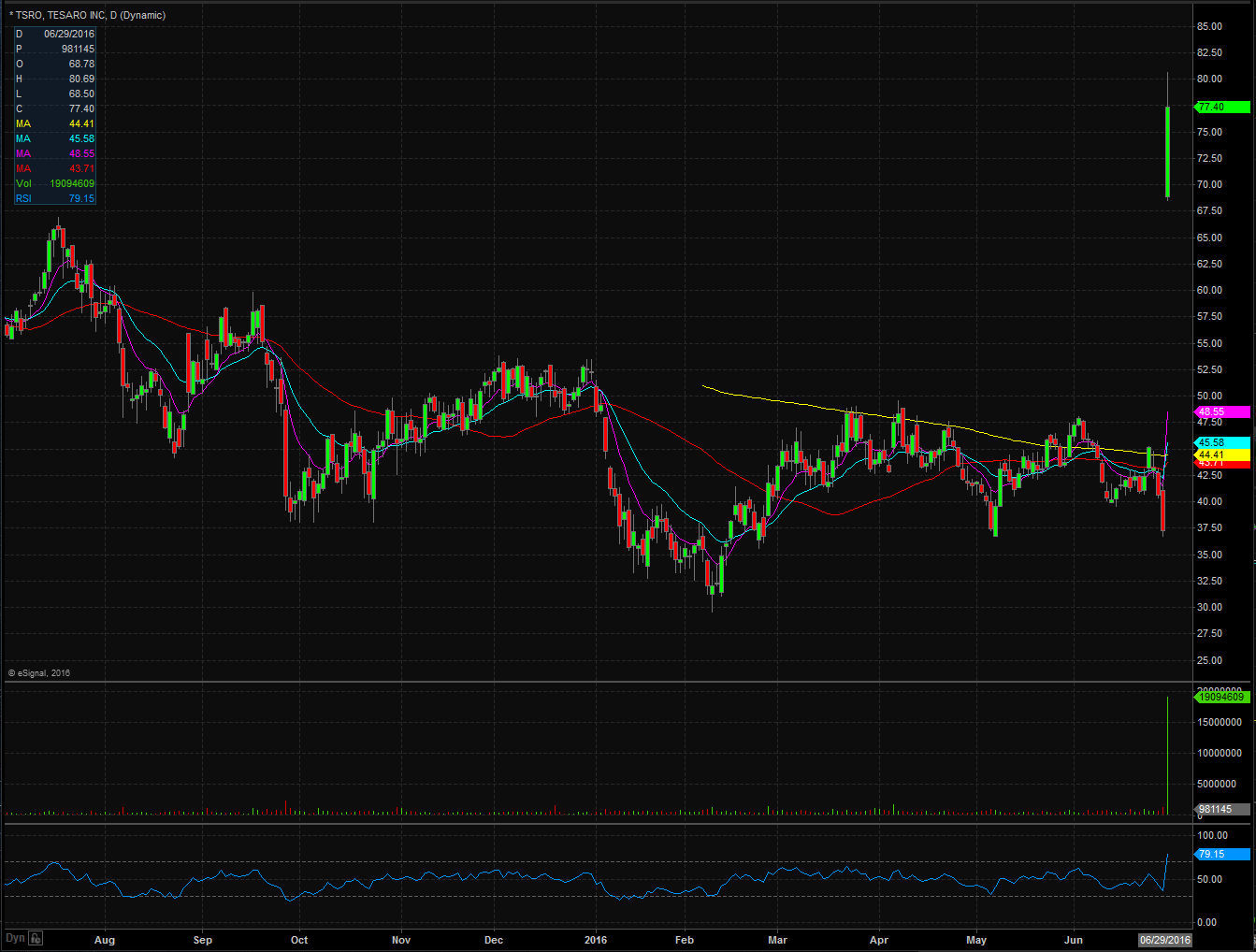
On Wednesday before the market opened, Tesaro announced that they had received positive data for its Phase-3 trials of its ovarian cancer treatment, Niraparib, as it achieved its primary endpoints of progression-free survival (PFS). TSRO plans to submit a new drug application and marketing authorization application for the fourth quarter of 2016. This is great news for the company and investors but even more important for the patients that could benefit from the use of this new treatment. Currently analysts have an average price target of $64.91 but that will most definitely be raised over the coming days following this news.
Looking at the chart you can see that shares absolutely skyrocketed after this positive data became public. Shares reached highs on Wednesday of $80.69 after closing Tuesday at $37.21 equaling a 116% jump in value! Shares ended the day at $77.40 after opening at $68.78 and were able to maintain that huge gap up showing that the buyers were in control. Another way to look at it is before the news came out, there was roughly a 41% short interest on the float meaning nearly half the float was short coming into this news according to YahooFinance. So we could have seen a lot of shorts covering their positions which held the stock up as well. Either way, this was a huge move which put shares at all time highs since they IPO’d back in 2012. We should see some support come in at the previous high of $66.95 and $64 while resistance will be met at whole dollar values since it is at new levels. Given that this was such a huge jump in prices I wouldn’t be surprised to see this come in a bit over the coming days.
COO Comments
We are extremely grateful to the patients, caregivers, and investigators who participated in the NOVA trial. The results of this study, which is the first successful, prospectively designed, randomized, well-controlled Phase 3 study of a PARP inhibitor, demonstrate that a single, daily, oral dose of niraparib is superior to control in prolonging PFS in women with recurrent ovarian cancer,” said Mary Lynne Hedley, Ph.D., President and COO of TESARO. “Importantly, these results show activity of niraparib in a population of ovarian cancer patients beyond those with germline BRCA mutations. In keeping with our mission of responsible drug development, NOVA was designed to define those patients most likely to benefit from niraparib treatment and, in so doing, optimize the benefit/risk profile for patients. We believe we have achieved that goal and look forward to presentation of the full data set from this study at the European Society for Medical Oncology (ESMO) congress in October.
TSRO Profile
TESARO, Inc., an oncology-focused biopharmaceutical company, identifies, acquires, develops, and commercializes cancer therapeutics and oncology supportive care products in the United States and internationally. Its product portfolio consist of Rolapitant, a neurokinin-1 receptor antagonist, which is in phase 1 intravenous clinical trials for the prevention of chemotherapy induced nausea and vomiting; Niraparib, an orally active and potent poly polymerase inhibitor to treat ovarian or breast cancers; and TSR-011, an anaplastic lymphoma kinase inhibitor, which is in phase 1/2a dose escalation clinical trial in cancer patients. The company also offers KEYTRUDA and OPDIVO, an anti-PD-1 antibody products, for the treatment of certain non-small cell lung cancers; and develops immunotherapy antibody product candidates targeting PD-1 (TSR-042) and TIM-3 (TSR-022), as well as LAG-3 and bi-specific antibody product candidates targeting PD-1/TIM-3, PD-1/LAG-3, and an additional bi-specific combination to treat various tumors. It has strategic research collaboration with Myriad Genetics, Inc. TESARO, Inc. was founded in 2010 and is headquartered in Waltham, Massachusetts. – YahooFinance
