Accelerating its growth strategy
SymBio (WA:SYM) recently acquired the exclusive rights to develop and market IONSYS, a fentanyl iontophoretic transdermal system for acute post-operative pain, in Japan. Treakisym is nearing a decision on additional indications and development is again moving ahead for rigosertib. With a third product in its development portfolio, we believe SymBio has the confidence to accelerate its in-licensing programme on its quest to become the pharmaceutical partner of choice in Asia-Pacific.
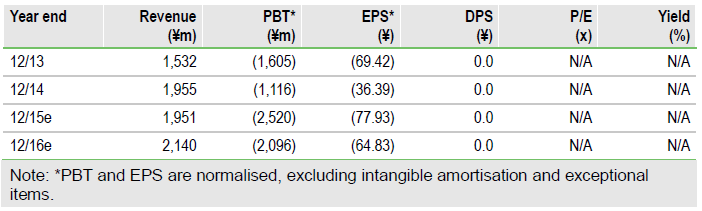
SymBio adds pain management to its portfolio
SymBio recently acquired exclusive rights to develop and market IONSYS, a patient-controlled fentanyl iontophoretic transdermal system in Japan, for $10m upfront plus undisclosed milestones and royalties. We believe IONSYS has a high probability of success – low regulatory risk, a large target market, positive clinical experience and clear competitive advantages over existing patient-controlled analgesia for post-operative pain based on a conventional electrical infusion pump. IONSYS diversifies, and in our view, de-risks SymBio’s development portfolio.
To Read the Entire Report Please Click on the pdf File Below