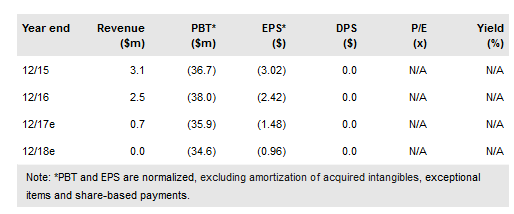
Sunesis Pharmaceuticals Inc (NASDAQ:SNSS) remains on track to complete the dose-escalation portion of its Phase Ib/II clinical trial for lead compound SNS-062, an oral Bruton’s tyrosine kinase (BTK) inhibitor, in patients with confirmed Imbruvica resistance in mid-2018, and present initial safety and efficacy interim data in Q218. In addition, Sunesis is moving forward with SNS-510, a PDK1 inhibitor with potential activity across multiple tumor types, and we expect a decision from Takeda on the advancement of pan-Raf inhibitor TAK-580 by mid- to late 2018. Sunesis completed a $20m offering of common and preferred stock in October 2017. We value Sunesis at $125.9m or $3.68.
SNS-062 Phase Ib/II readout approaching
The first data on the efficacy of SNS-062 is expected as part of the interim readout from the dosing portion of the Phase Ib/II trial. The data are planned to be presented at a major conference in mid-2018. Sunesis will also provide an update on the program at the American Society of Hematology (ASH) conference in December 2017.
To read the entire report Please click on the pdf File Below: