Shire plc (NASDAQ:SHPG) announced positive top-line results from the phase III study, HELP, on pipeline candidate, lanadelumab.
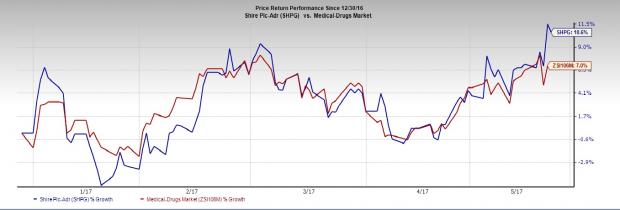
Shire’s shares have rallied 10.6% so far this year, outperforming the Zacks classified Medical-Drugs industry’s 7.0% rise.
The global, multi-center, randomized, double-blind, placebo-controlled parallel group trial evaluated the efficacy and safety of subcutaneously administered lanadelumab versus placebo over 26 weeks in patients 12 years of age or older with Hereditary Angioedema (HAE).
The study met its primary endpoint and all secondary endpoints with highly statistically significant and clinically meaningful results for all three lanadelumab treatment arms compared to placebo.
The study results showed that 300 mg dose administered once every two weeks resulted in a statistically significant reduction in mean HAE attack frequency of 87% compared to placebo (p
Consequently, Shire plans to submit a biologics license application (BLA) to the FDA by late 2017 or early 2018. We note that lanadelumab enjoys both Orphan Drug Designation and Breakthrough Therapy Designation in the U.S. and Orphan Drug Designation in the EU. Following the news, shares moved up 7.8% in session.
The successful development and commercialization of lanadelumab will boost Shire’s HAE portfolio which currently boasts Cinrzye and Firazyr. Shire had earlier acquired the biotech company, Dyax Corp., to strengthen its position in the HAE market further. The acquisition added an approved HAE treatment, Kalbitor, to Shire’s portfolio.
Concurrently, Shire announced that results from a phase II study (NCT01620255) investigating an anti-mucosal addressin cell adhesion molecule 1 (MAdCAM-1) antibody SHP647 (formerly PF-00547659) for the treatment of moderate-to-severe ulcerative colitis in adults were published in The Lancet.
The results from the phase II study showed that candidate met its primary endpoint demonstrating significantly greater remission rates in patients receiving anti-MAdCAM antibody compared to placebo in three of four tested dose groups.
Shire plans to initiate a phase III trial on SHP647 in the second half of 2017. We note thet Shire had licensed the global rights to all indications for SHP647 from Pfizer Inc. (NYSE:PFE) in Jun 2016 to strengthen its GI portfolio. Shire’s efforts to develop its GI franchise are encouraging. The Feb 2015 NPS Pharma acquisition (worth $5.2 billion) added Gattex/Revestive (short bowel syndrome) and Natpara/Natpar (an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathyroidism) to Shire’s portfolio.
Zacks Rank & Key Picks
Shire currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the health care sector include VIVUS, Inc. (NASDAQ:VVUS) , and Aeglea BioTherapeutics (NASDAQ:AGLE) . While VIVUS sports a Zacks Rank #1 (Strong Buy), Aeglea carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
VIVUS’s loss per share estimates narrowed from 50 cents to 39 cents for 2017, over the last 30 days. The company posted positive earnings surprises in all of the four trailing quarters with an average beat of 233.69%.
Aeglea’s loss per share estimates narrowed from $3.64 to $2.48 for 2017, over the last 60 days. The company posted positive earnings surprises in three of the four trailing quarters with an average beat of 20.75%.
Zacks' Hidden Trades
While we share many recommendations and ideas with the public, certain moves are hidden from everyone but selected members of our portfolio services. Would you like to peek behind the curtain today and view them?
Starting now, for the next month, I invite you to follow all Zacks' private buys and sells in real time from value to momentum...from stocks under $10 to ETF to option movers...from insider trades to companies that are about to report positive earnings surprises (we've called them with 80%+ accuracy). You can even look inside portfolios so exclusive that they are normally closed to new investors. Click here for Zacks' secret trade>>
Pfizer, Inc. (PFE): Free Stock Analysis Report
VIVUS, Inc. (VVUS): Free Stock Analysis Report
Aeglea BioTherapeutics, Inc. (AGLE): Free Stock Analysis Report
Shire PLC (SHPG): Free Stock Analysis Report
Original post
Zacks Investment Research
