Sanofi (PA:SASY) (NASDAQ:SNY) announced that a phase III study, evaluating its pipeline candidate — isatuximab — for the treatment of patients with relapsed/refractory multiple myeloma, met the primary endpoint of prolonging progression free survival for the given patient population.
The study was examining isatuximab, an anti-CD38 monoclonal antibody plus standard of care medicines, namely pomalidomide and lowdose dexamethasone compared with pomalidomide and low-dose dexamethasone alone.
The open label phase III ICARIA-MM program enrolled 307 patients suffering relapsed/refractory multiple myeloma, who received two or more prior anti-myeloma therapies. The safety profile of isatuximab was the study’s secondary endpoint.
Data from this study will be submitted at an upcoming medical meeting and is expected to support the regulatory filling for the candidate, which is planned later this year.
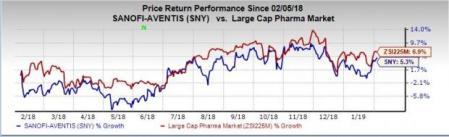
Shares of Sanofi have increased 5.3% in the past year compared with the industry’s rise of 6.9%.
Notably, Sanofi is focusing on its R&D efforts for key technologies and diseases, which hold greater commercial potential. At the end of October 2018, Sanofi’s pipeline included 40 pharmaceutical new molecular entities and vaccine candidates, which were in phase III analyses or under regulatory review.
Apart from isatuximab, Sanofi’s portfolio has another promising candidate, which is cemiplimab. It is being evaluated for several cancer indications. In September 2018, Libtayo/cemiplimab was approved in the United States for treating cutaneous squamous cell carcinoma while an approval in the EU is awaited by the first half of 2019. The drug is also being investigated for multiple other cancers including potentially pivotal trials in lung, cervical and skin cancers.
Sanofi has entered into a development and commercialization partnership agreement with Regeneron Pharmaceuticals (NASDAQ:REGN) for Libtayo.
Zacks Rank & Stocks to Consider
Sanofi currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the large cap pharma sector include Novo Nordisk (CO:NOVOb) A/S (NYSE:NVO) and Eli Lilly and Company (NYSE:LLY) . While Novo Nordisk sports a Zacks Rank #1 (Strong Buy), Eli Lilly carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Novo Nordisk’s earnings estimates have been revised 1.9% upward for 2019 over the past 60 days.
Eli Lilly’s earnings estimates have moved 1.4% north for 2019 over the past 60 days. The stock has soared 55.1% in a year.
Zacks' Top 10 Stocks for 2019
In addition to the stocks discussed above, would you like to know about our 10 finest buy-and-holds for the year?
Who wouldn't? Our annual Top 10s have beaten the market with amazing regularity. In 2018, while the market dropped -5.2%, the portfolio scored well into double-digits overall with individual stocks rising as high as +61.5%. And from 2012-2017, while the market boomed +126.3, Zacks' Top 10s reached an even more sensational +181.9%.
Sanofi (SNY): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Novo Nordisk A/S (NVO): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Get Free Report
Original post
Zacks Investment Research
