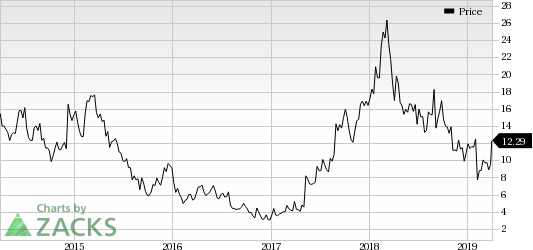
Sangamo Therapeutics, Inc. (NASDAQ:SGMO) and its partner Pfizer (NYSE:PFE) announced encouraging interim data from the phase I/II Alta study evaluating their gene therapy candidate, SB-525, in patients with severe hemophilia A. Data showed that the gene therapy candidate holds potential.
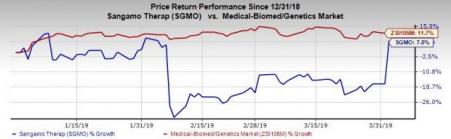
Shares of Sangamo rallied almost 28% on Apr 2 following the data readout. However, shares of the company have increased 7% so far this year compared with the industry’s rise of 11.7%.
The phase I/II study evaluated four doses of SB-525 – 9e11 vg/kg, 2e12 vg/kg, 1e13 vg/kg and 3e13 vg/kg – with two patients per cohort. Data from the study showed that patients treated with the highest dosage (3e13 vg/kg) achieved normal factor VIII levels. Dose dependent increase in factor VIII levels were observed in the eight patients treated with SB-525 gene therapy across the four dosage cohorts. Factor VIII boosts blood clotting, which is a major symptom of haemophilia. Moreover, the candidate was well tolerated in every dosage cohort.
The company believes that SB-525 may achieve predictability and sustained treatment effect, which can lead to clinical benefit in haemophilia A patients.
Based on the data from the study, the Safety Monitoring Committee ("SMC") recommended expansion of the 3e13 vg/kg cohort. Total patients in the cohort will increase to five. Longer-term follow up data will be presented at a future scientific meeting.
Although interim data from the study seems promising, the clinical development of SB-525 is in early stage with commercialization prospects years away.
We note that there are other treatments available for treating hemophilia A and several others are in development including gene therapies. In February 2019, Novo Nordisk’s (NYSE:NVO) Esperoct was approved for the indication. Companies developing gene therapy candidates for treating hemophilia A include Spark Therapeutics (NASDAQ:ONCE) and uniQure. The most advanced gene therapy for hemophilia A treatment in clinical studies is BioMarin’s valoctocogene roxaparvovec (BMN-270). Late-stage studies are evaluating 6e13 vg/kg and 4e13 vg/kg doses of the candidate.
Apart from SB-525, Sangamo is also developing gene therapies in collaboration with Pfizer for treating amyotrophic lateral sclerosis and frontotemporal lobar degeneration.
Zacks Rank
Sangamo currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Is Your Investment Advisor Fumbling Your Financial Future?
See how you can more effectively safeguard your retirement with a new Special Report, “4 Warning Signs Your Investment Advisor Might Be Sabotaging Your Financial Future.”
Novo Nordisk (CO:NOVOb) A/S (NVO): Free Stock Analysis Report
Pfizer Inc. (PFE): Free Stock Analysis Report
Spark Therapeutics, Inc. (ONCE): Free Stock Analysis Report
Sangamo Therapeutics, Inc. (SGMO): Free Stock Analysis Report
Original post