Roche Holdings AG (OTC:RHHBY) announced that the FDA has granted Breakthrough Therapy Designation for its experimental drug oral medicine balovaptan (previously known as RG7314), a vasopressin 1a (V1a) receptor antagonist for individuals with autism spectrum disorder (ASD).
The designation from the FDA is intended to expedite the development and review of drugs with early evidence of substantial potential clinical benefit to patients, or benefit patients without current treatment options.
The designation for balovaptan is primarily based on efficacy findings in the phase II trial, VANILLA (Vasopressin ANtagonist to Improve sociaL communication in Autism) study, in adults with ASD.
Meanwhile, a phase II trial balovaptan in children and adolescents with ASD is ongoing and other trials in ASD are also being planned.
We note that ASD is approximately four times more prevalent in boys than in girls. As per estimates, 1 in 42 boys and 1 in 189 girls suffer from ASD in the United States. The World Health Organization estimates that the global prevalence of ASD is approximately one in every 160 people and 0.3% of the global burden of disease.
Roche has more than a dozen pipeline candidates in the neuroscience portfolio for diseases that include multiple sclerosis, Alzheimer’s disease, spinal muscular atrophy, Parkinson’s disease and autism.
Earlier in the month, the European Commission granted marketing authorization to multiple sclerosis drug Ocrevus. The approval boosts Roche’s neuroscience portfolio.
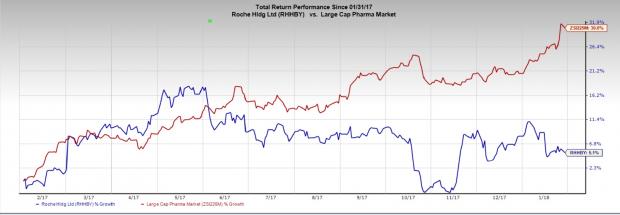
Roche’s stock has rallied 5.1% over a year compared with industry's gain of 30.6%.
Approval of new drugs and a potential label expansion of existing drugs bode well for Roche as its legacy drugs like Herceptin, MabThera are facing competition from biosimilars.
Novartis AG (NYSE:NVS) has already launched its biosimilar version of Rituxan/ MabThera in Europe. Amgen (NASDAQ:AMGN) has also obtained FDA approval for a biosimilar version of Avastin for treatment of five types of cancers including lung cancer, colorectal cancer, glioblastoma, renal cell carcinoma and cervix cancer.
Zacks Rank & Key Pick
Roche carries a Zacks Rank #3 (Hold).
A better-ranked stock in the healthcare sector is Exelixis, Inc. (NASDAQ:EXEL) with a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Exelixis’ earnings per share estimates have moved up from 72 cents to 77 cents for 2018 over the last 60 days. The company delivered a positive earnings surprise in the last four quarters, with an average beat of 572.92%.
Don’t Even Think About Buying Bitcoin Until You Read This
The most popular cryptocurrency skyrocketed last year, giving some investors the chance to bank 20X returns or even more. Those gains, however, came with serious volatility and risk. Bitcoin sank 25% or more 3 times in 2017.
Zacks has just released a new Special Report to help readers capitalize on the explosive profit potential of Bitcoin and the other cryptocurrencies with significantly less volatility than buying them directly.
See 4 crypto-related stocks now >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Amgen Inc. (AMGN): Free Stock Analysis Report
Exelixis, Inc. (EXEL): Free Stock Analysis Report
Original post
Zacks Investment Research
