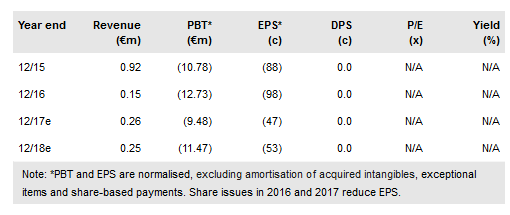
Txcell (PA:TXCL) has disclosed that it has a viable manufacturing route for its novel CAR Treg products. This uses a stable but low-frequency Treg cell type. The TxCell methodology uses a robust design to give low inter-patient variability with potentially consistent therapeutic results. Regulatory filings for a dose-ranging clinical trial are expected by late 2018. On the financial side, TxCell will need to draw at least €10m of a new, less onerous set of convertible loans to support CAR Treg development. The indicative valuation has been increased to €87.9m from €84.4m as probabilities have been slightly adjusted. Cash on 31 December 2017 was €4.9m.
Focus on CAR Treg with multiple preclinical projects
TxCell is set on being the leading Treg company to treat immune disorders using chimeric antigen receptor (CAR) technology; this is similar to that used in the cancer T-cell area. The design of a humanised CAR Treg plus the announcement of a robust manufacturing process have been key development stages. CAR Treg trials as a proof of concept are planned in solid organ transplant rejection. Other projects are at an earlier-stage of research; these could target broader markets.
To read the entire report Please click on the pdf File Below: