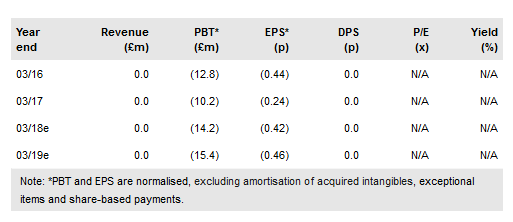
Reneuron Group's (LON:RQE) interim results for the six months to 30 September 2017 showcased a crucial juncture in the company’s history. With the first of two US placebo-controlled, late-stage studies in chronic stroke starting in 2018 and dosing ongoing in the US Phase I/II study in retinitis pigmentosa, ReNeuron’s stem cell therapies are aimed at helping the disabled walk and the blind see. ReNeuron reported a loss of £9.6m for the six months (vs £7.7m in H117). In 2018 ReNeuron’s profile will increase further as it conducts two clinical studies and opens an operational base in the US.
First late-stage study in chronic stroke starts
ReNeuron will start dosing chronic stroke patients in a randomized, placebo-controlled study of its CTX cells in early 2018. An open investigational new drug (IND) application brings ReNeuron unprecedented exposure in the US, which will be aided by its recently announced opening of a US office. When the FDA allows a biotechnology company to start a US clinical study, it has already been through considerable scrutiny by the regulator and the progress of its studies can be followed on www.clinicaltrials.gov. This means that potential partners can keep a close eye on ReNeuron’s progress and determine when the PISCES III study has completed enrolment, for example.
To read the entire report Please click on the pdf File Below: