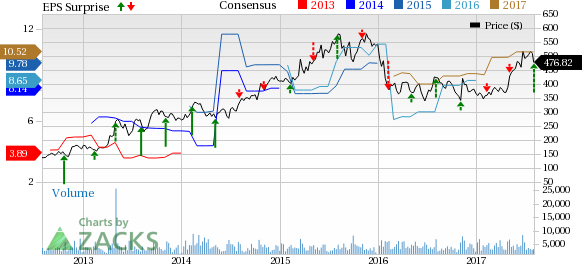
Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) reported second-quarter of 2017 results wherein both earnings and sales beat expectations on the back of strong performance of eye-care drug Eylea.
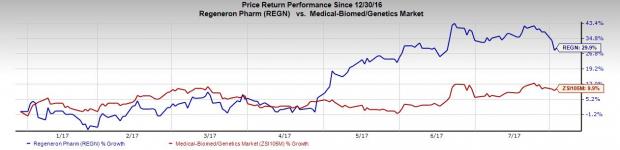
Regeneron’s stock has gained 29.9% year to date outperforming the industry’s 9.9% rally. Shares are up in pre-market trading and we expect shares to gain further momentum.
The company reported earnings (including the impact of share-based compensation expenses and tax adjustments) of $3.88 per share in the second-quarter beating the Zacks Consensus Estimate of $2.67.
Excluding share-based compensation expenses, Regeneron’s earnings came in at $4.17 per share, up from $2.82 reported in the year-ago quarter.
Total revenue in the second quarter increased 21.2% year over year to $1.47 billion driven by strong sales of eye treatment drug, Eylea. Revenues were above the Zacks Consensus Estimate of $1.36 billion.
We note Regeneron has co-developed Eylea with the HealthCare unit of Bayer (DE:BAYGN) AG (OTC:BAYRY) . The company is solely responsible for the U.S. sales of the eye drug and is entitled to profits. However, it shares profits and losses equally with Bayer from ex-U.S. Eylea sales, except in Japan, where the company receives a royalty on net sales.
Quarterly Highlights
Net product sales increased to $924.1 million in the reported quarter, up 10.8% year over year. The majority of sales came from Eylea in the U.S. ($919 million, up 10.6%). Sales of Eylea in ex-U.S. markets were $542 million, up from $486 million reported in the year-ago quarter.
Revenues also include Sanofi (NYSE:SNY) and Bayer collaboration revenues of $432 million, compared with $355 million in the year-ago quarter. Collaboration revenues from Sanofi were $222.1 million in the quarter, compared with $163.4 million a year ago. Praluent recorded global net sales of $46 million in the reported quarter, up from $24 in the year-ago quarter. We note Praluent has been co-developed in collaboration with Sanofi. Product sales for Praluent are recorded by Sanofi, while
Regeneron shares profits or losses from the commercialization of the drug.
Dupixent sales came in at $29 million. The drug was approved earlier in 2017 for the treatment of adults with moderate-to-severe atopic dermatitis (AD).
R&D expenses decreased 8.9% while selling, general and administrative (SG&A) increased 5.1% during the quarter.
2017 Outlook Updated
Based on a strong first half, the company raised its sales guidance for Eylea. In 2017, Regeneron expects U.S. Eylea net sales to grow around 10% (earlier guidance: growth in single digits). The company now expects adjusted unreimbursed
R&D expenses in the range of $925–$965 million, down from the earlier guidance of $950–$1,025 million.
We note that the European Medicine Agency's Committee for Medicinal Products for Human Use (CHMP) rendered a positive opinion for the marketing authorization of Dupixent, recommending its approval for use in adults with moderate-to-severe atopic dermatitis who are candidates for systemic therapy in Jul 2017. Meanwhile, a phase III study of Dupixent in pediatric patients (6-11 years of age) with uncontrolled persistent asthma was initiated in second-quarter 2017.
In May 2017, the FDA approved Kevzara for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have an inadequate response or intolerance to one or more disease modifying anti-rheumatic drugs (DMARDs). The drug was also approved in Europe in Jun 2017.
In Jun 2017, the European Commission granted marketing authorization for Kevzara in combination with methotrexate (MTX) for the treatment of moderately to severely active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more DMARDs; Kevzara may be used as monotherapy in case of intolerance to MTX or when treatment with MTX is inappropriate.
Our Take
Regeneron’s second-quarter results were impressive as both earnings and sales beat estimates driven by strong Eylea sales. The increase in guidance was also encouraging. Dupixent launch in the U.S. for moderate-to-severe atopic dermatitisis progressing well and a potential approval in the EU in the second half of 2017 will further boost sales. Moreover, the company is also looking to expand Dupixent’s label in uncontrolled asthma.
Zacks Rank& Key Pick
Regeneron currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the healthcare sector is Gilead Sciences. Inc. (NASDAQ:GILD) which currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Gilead’s earnings per share estimates increased from $7.92 to $8.53 for 2017, over last thirty days following strong results in the second quarter. The company delivered positive earnings surprises in three of the trailing four quarters, with an average beat of 8.18%.
More Stock News: Tech Opportunity Worth $386 Billion in 2017
From driverless cars to artificial intelligence, we've seen an unsurpassed growth of high-tech products in recent months. Yesterday's science-fiction is becoming today's reality. Despite all the innovation, there is a single component no tech company can survive without.
Demand for this critical device will reach $387 billion this year alone, and it's likely to grow even faster in the future. Zacks has released a brand-new Special Report to help you take advantage of this exciting investment opportunity. Most importantly, it reveals 4 stocks with massive profit potential. See these stocks now>>
Sanofi (SNY): Free Stock Analysis Report
Bayer AG (BAYRY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Gilead Sciences, Inc. (GILD): Free Stock Analysis Report
Original post
Zacks Investment Research