Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) and partner Sanofi (NYSE:SNY) announced positive results related to its experimental candidate, REGN2810, a checkpoint inhibitor targeting PD-1 (programmed death 1) in patients with advanced cutaneous squamous cell carcinoma (CSCC). The data was presented at the 2017 American Society of Clinical Oncology Annual meeting.
The data from two cohorts of a phase I study demonstrated that treatment with REGN2810 achieved overall response rate (ORR) of 46.2% and a disease control rate (DCR) of 69.2%. However, the median progression free survival and overall survival is yet to be reached.
The phase I study evaluated REGN2810 with an initial dose-escalation portion, followed by multiple expansion cohorts to investigate safety and antitumor activity in specific patient populations. The data presented were from two cohorts evaluating the candidate in patients with distantly metastatic CSCC and with inoperable (unresectable) locally or regionally advanced CSCC. A 3mg/kg dose was administered through intravenous infusion over 30 minutes every two weeks for up to 48 weeks.
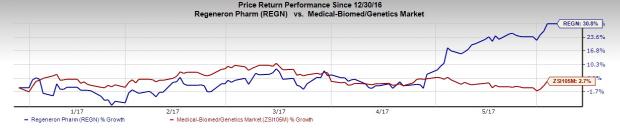
So far this year, Regeneron’s share price has risen 30.8%, significantly better than a 2.7% increase for the Zacks classified Medical - Biomedical and Genetics industry.
Another expansion cohort of the phase I study showed no apparent association between the objective response and level of PD-L1 in patients. Other correlative studies are ongoing. The candidate is being developed as monotherapy for CSCC, basal cell carcinoma (BCC) and non-small cell lung cancer (NSCLC). A combination therapy of REGN2810 and REGN3767 is also being developed as an immunotherapy targeting the checkpoint inhibitor LAG-3.
Another phase II study, EMPOWER-CSCC 1, is evaluating REGN2810 in advanced CSCC and is currently in the enrolment phase.
We remind investors that the FDA has approved Dupixent and Kevzara earlier this year for atopic dermatitis and rheumatoid arthritis, respectively.
The company is focused on developing its investigational therapies and has collaborations with Intellia Therapeutics Inc. (NASDAQ:NTLA) for accessing Intellia's CRISPR/Cas gene-editing technology platform and with Teva Pharmaceutical Industries Limited (NYSE:TEVA) for the development and commercialization of nerve growth factor (NGF) antibody candidate, fasinumab globally.
Currently, Regeneron Pharma sports a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Looking for Stocks with Skyrocketing Upside?
Zacks has just released a Special Report on the booming investment opportunities of legal marijuana. Ignited by new referendums and legislation, this industry is expected to blast from an already robust $6.7 billion to $20.2 billion in 2021. Early investors stand to make a killing, but you have to be ready to act and know just where to look. See the pot trades we're targeting>>
Sanofi (SNY): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Intellia Therapeutics, Inc. (NTLA): Free Stock Analysis Report
Teva Pharmaceutical Industries Limited (TEVA): Free Stock Analysis Report
Original post
