ProNAi Therapeutics Inc. (NASDAQ:DNAI)
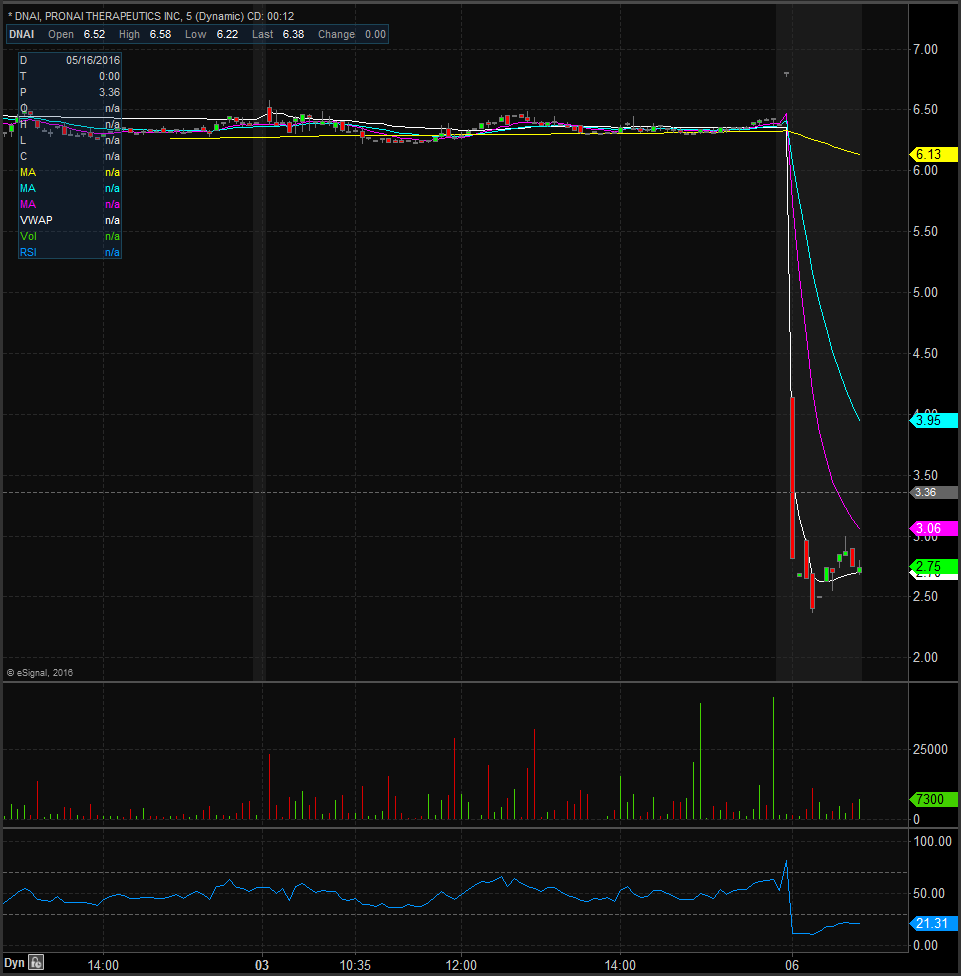
On Monday morning before the market opened, ProNai Therapeutics announced that it will discontinue the development of its lymphoma treatment after it experienced disappointing Phase-2 trials results that were not robust enough to justify the continuation of its development. Looking at the daily chart you will see that shares have been under some heavy selling pressure dating back to September of last year and after this news shares are looking to hit all time lows. Shares are currently trading at $2.67 after closing Friday at $6.38 equivalent to a 58% drop in price. There should be some support at the whole and half dollar values so we’ll have to see how shares act at $2.50 and $2 for support and resistance will come in at $4.50 and $5.50. Currently analysts have an average price target of $27.67 but this news could have a negative impact on it.
Executive Comments
Although we observed modest efficacy from PNT2258 in this interim analysis of Wolverine, we do not view these results as robust enough to justify continued development of the drug in DLBCL. We have decided to suspend development of PNT2258 pending further review of these data in order to determine next steps for both this asset and the DNAi platform,” said Dr. Nick Glover, President and CEO of ProNAi. “We continue to maintain a strong balance sheet and will focus our resources and activities on advancing our newly licensed Cdc7 inhibitor, PNT141, as well as on securing additional assets to build a broad and diverse pipeline of oncology drugs under our development.
We designed and conducted a robust, well-executed set of experiments, both clinical and preclinical, in order to further our understanding of the PNT2258 asset and the underlying DNAi technology. Unfortunately, advanced DLBCL and Richter’s Transformation are challenging diseases to treat, and PNT2258 did not markedly improve outcomes in these indications,” said Dr. Barbara Klencke, Chief Development Officer of ProNAi. “On the basis of these interim assessments, we have decided to close the Wolverine and Brighton studies to further enrollment of new subjects. On behalf of ProNAi, we would like to thank the patients and their families, investigators and staff involved in these studies for their participation and support.
DNAI Profile
ProNAi Therapeutics, Inc., a clinical-stage oncology company, develops and commercializes drugs based on its DNA interference (DNAi) technology platform for patients with cancer and hematological malignancies. Its lead DNAi product candidate is PNT2258, a proprietary formulation of PNT100, a single-stranded 24-base DNAi oligonucleotide, which is in Phase II clinical trials for the treatment of relapsed or refractory diffuse large B-cell lymphoma; and Richter’s transformed chronic lymphocytic leukemia. The company was formerly known as Phenome Systems, Inc. and changed its name to ProNAi Therapeutics, Inc. in April 2004. ProNAi Therapeutics, Inc. was founded in 2003 and is headquartered in Vancouver, Canada.