Operating costs in H117 were largely in line with our expectations, while a one-off payment was a welcome resolution of Probiodrug’s long-outstanding potential tax liability. Following the overall positive Phase IIa data with PQ912 for Alzheimer’s disease (AD) released in June 2017, Probiodrug AG (AS:PDB) reiterated in its H117 report that all strategic options for further development are on the table.
Depending on available funding, the company could initiate the next study looking at the long-term treatment of AD patients (the Phase IIa SPAHIR treatment period was three months) or, if a suitable partner emerges, a deal could alleviate the late stage PQ912 development. Our valuation has increased slightly to €496m or €61/share.
All options on table for further PQ912 development
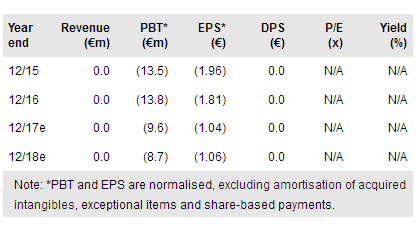
Probiodrug reported R&D and G&A costs of €4.9m and €1.3m respectively in H117 compared to €4.7m and €1.3m in H116. These results are largely in line with our expectations; our FY17 R&D estimate stands at €7.7m implying H217 should be less costly, as the results from the Phase IIa SAPHIR trial (main cost driver) were announced in June 2017. Probiodrug reported cash of €14.4m (no debt) versus €21.9m at end FY16.
Cash reach and additional funding requirements for the near term depend on the PQ912 development strategy. Probiodrug has indicated that it is open to exploring all options from running the next proof-of-concept trial on its own to establishing a partnership. Input from the full analysis of the SAPHIR trial will be important for the design of the next trial and is due in several months.
To read the entire report please click on the pdf file below: