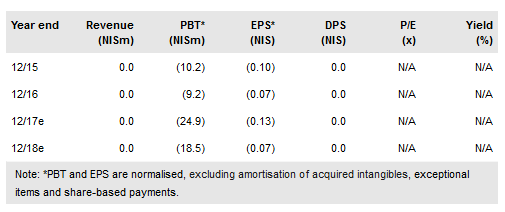
Over 2017 BiondVax Pharmaceuticals Ltd ADR (NASDAQ:BVXV) crystallised its late-stage development plans for lead asset M-001, a potentially universal influenza vaccine. Following its discussions with the regulatory authorities and gaining access to new capital (European Investment Bank and share issues), the company confirmed on 27 December 2017 that it will initiate a pivotal Phase III trial on its own for a universal flu vaccine indication (likely to start in Q318). We have revised our assumptions substantially and increased our valuation to $200m (NIS689m) or $32.4/ADS (NIS2.80/share) from $165m previously.
M-001 as a universal, standalone vaccine
BiondVax now plans to move directly into Phase III with M-001 as a standalone influenza vaccine. The company has explored various paths to the market, including targeting smaller populations such as a pandemic primer for stockpiling or a seasonal primer for populations at risk. After the consultation with the regulatory authorities and gaining access to the €20m loan from the European Investment Bank (EIB), BiondVax attracted new equity investments and now is preparing the Phase III trial for the ultimate goal of developing a standalone universal influenza vaccine.
To read the entire report Please click on the pdf File Below: