Pfizer Inc. (NYSE:PFE) and partner Avillion announced that a supplemental New Drug Application (sNDA) for Bosulif has been accepted and granted priority review by the FDA.
We remind investors that Bosulif is currently approved for the treatment of chronic, accelerated or blast phase Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML) in adults who are resistant or intolerant to prior therapy.
The company is looking to get the drug approved for the first-line treatment of patients suffering from chronic phase Ph+ CML. A decision from the FDA is expected in December 2017.
Additionally, the European Medicines Agency (EMA) has also accepted a similar regulatory application for review.
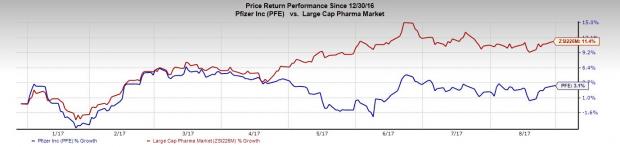
Pfizer shares have gained 3.1% so far this year, underperforming the industry’s gain of 11.4% in the same period.
The application also includes patients who are not eligible for treatment with Novartis AG’s (NYSE:NVS) Gleevec or Tasigna and Bristol-Myers Squibb Company’s (NYSE:BMY) Sprycel.
The regulatory application in the U.S. and EU based on data from the phase III BFORE study, which evaluated a 400 mg dose of Bosulif in newly diagnosed Ph+ CML patients. The results showed that Bosulif achieved major molecular response at 12 months in significantly higher number of patients compared to Gleevec.
Per the press release, there are 48,000 patients with CML in the U.S. and 9,000 new cases are expected to be diagnosed in 2017. This represents an opportunity for Bosulif if approved as first-line treatment for Ph+ CML.
Pfizer is expecting a decision from the FDA on label expansion of another leukemia drug, Mylotarg in first-line setting for treating acute myeloid leukemia in September 2017. In July, the FDA’s advisory panel had voted in favor of approval of the sNDA for Mylotarg.
Zacks Rank & Stock to Consider
Pfizer has a Zacks Rank #3 (Hold).
A better-ranked stock in the pharma sector is Corcept Therapeutics Incorporated (NASDAQ:CORT) , which carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Corcept’s earnings estimates increased from 26 cents to 42 cents for 2017 and from 49 cents to 70 cents for 2018 over the last 30 days. The company delivered an average earnings beat of 29.17% for the four trailing quarters. The stock is up 108% so far this year.
4 Surprising Tech Stocks to Keep an Eye On
Tech stocks have been a major force behind the market’s record highs, but picking the best ones to buy can be tough. There’s a simple way to invest in the success of the entire sector. Zacks has just released a Special Report revealing one thing tech companies literally cannot function without. More importantly, it reveals 4 top stocks set to skyrocket on increasing demand for these devices. I encourage you to get the report now – before the next wave of innovations really takes off.
Bristol-Myers Squibb Company (BMY): Free Stock Analysis Report
Pfizer, Inc. (PFE): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT): Free Stock Analysis Report
Original post
Zacks Investment Research