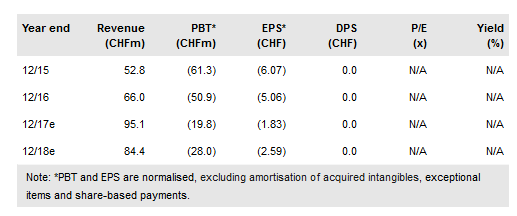
Basilea has announced an extension to its licence agreement with Pfizer (NYSE:PFE) for Cresemba (for invasive fungal infections). The original deal granted PFE exclusive commercialisation rights to the product in Europe (ex-Nordics), Russia, Turkey and Israel and the new amendment includes China and 16 countries within Asia Pacific. The extension of Cresemba’s global footprint at this stage highlights PFE’s commitment to the product in areas outside of Europe. Concomitantly Basilea has updated its guidance for FY17 to a reduced operating loss of CHF1m per month vs CHF2m per month. Our valuation rises slightly to CHF1,222m.
PFE to commercialise Cresemba in China & Asia Pac
Pfizer is to use its extensive footprint in China and Asia Pac to commercialise Cresemba (isavuconazole) contingent on approval in each territory. The revised agreement includes a $3m upfront payment (deferred) and up to ~$223m in additional payments dependent upon the achievement of pre-specified regulatory and sales milestones. Basilea will also receive royalties in the mid-teen range on Pfizer’s sales in these territories. Importantly, as of November 2017 PFE has launched Cresemba into Spain so it is now available in the top five EU markets. In addition, Swissmedic has granted marketing authorisation for Switzerland.
To read the entire report Please click on the pdf File Below: