A new drug application (“NDA”) for Pfizer’s (NYSE:PFE) anaplastic lymphoma kinase (“ALK”) tyrosine kinase inhibitor (“TKI”) candidate, lorlatinib has been accepted by the FDA. The NDA sought approval of lorlatinib for the treatment of patients ALK-positive metastatic non-small cell lung cancer (“NSCLC”) who have received prior treatment with one or more ALK TKIs. The NDA has also been granted priority review. A decision is expected in August 2018.
Similar applications seeking marketing authorization in Europe and Japan have also been accepted by the regulatory agencies of the respective countries.
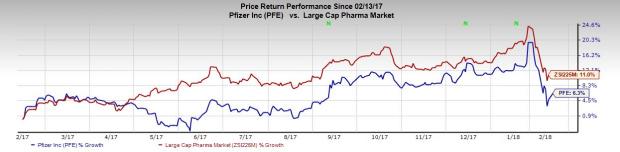
Shares of Pfizer were up almost 1.5% following the news. The stock has returned 6.3% in the past year, underperforming the industry’s gain of 11% in that period.
Pfizer submitted the regulatory applications based on positive data from phase II portion of a phase I/II study, evaluating lorlatinib in several cohorts based on prior therapies.
Non-small cell lung cancer occurs in 85% of the patients who have lung cancer, the leading cause of cancer death worldwide.
Lung cancer is an attractive avenue for pharmaceutical companies due to the widespread occurrence of the disease. However, it is also one of the most competitive spaces due to the presence of a large number of players. Several large and small companies are either developing or have approved therapies for the treatment of various forms of lung cancer.
We remind investors that last month Merck (NYSE:MRK) announced encouraging results from a phase III study evaluating its anti-PD-1 therapy, Keytruda, in combination with Eli Lilly’s (NYSE:LLY) Alimta and carboplatin in NSCLC. We also note that Roche (OTC:RHHBY) recently presented data on Tecentriq in novel combinations across a broad range of tumors including lung cancer.
Blockbuster drugs like Bristol-Myers’ Opdivo is approved for treating NSCLC, among others.
Zacks Rank
Pfizer carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Roche Holding (SIX:ROG) AG (RHHBY): Free Stock Analysis Report
Pfizer, Inc. (PFE): Free Stock Analysis Report
Eli Lilly and Company (LLY): Free Stock Analysis Report
Merck & Company, Inc. (MRK): Free Stock Analysis Report
Original post
Zacks Investment Research