Paratek Pharmaceuticals (NASDAQ:PRTK), a clinical stage biopharmaceutical company yesterday announced that their antibiotic drug omadacycline, which is used for the treatment of Acute bacterial skin and skin infections met all the endpoints in its FDA study. The phase-3 study showed that omadacycline met all its primary and secondary efficacy outcomes designated by the Food and Drug Administration (FDA) and European Medicines Agency (EMA).
Paratek Pharmaceuticals, Inc. CEO and Chief Medical Officer Comments
The successful achievement of these primary efficacy and secondary outcomes, combined with the safety and tolerability outcomes for both the oral and IV formulations of omadacycline is a significant step towards securing regulatory approval and advancing omadacycline to commercialization,” said Michael Bigham, Chairman and Chief Executive Officer of Paratek. “Increasingly, patients and physicians are faced with the growing challenge that existing antibiotic therapies are failing as pathogens develop resistance. The positive data from this registration study demonstrate the clear potential of omadacycline to treat serious community-acquired infections where resistance is of concern.
These Phase 3 data are highly encouraging and continue to support our belief and confidence in the efficacy and safety profile of omadacycline, which has now been evaluated in more than 1,000 subjects in clinical trials. We believe omadacycline has the potential to provide physicians with an important new well-tolerated, broad spectrum, once-daily, oral and IV antibiotic to treat serious, often life-threatening, community-acquired infections.” said Evan Loh, M.D., President and Chief Medical Officer. “The successful completion of our first registration study is a major milestone for Paratek and we are grateful to the patients, investigators, the dedicated Paratek team, and our partners for their efforts in helping us advance the development of omadacycline,” Globe Newswire
PRTK Technical Analysis
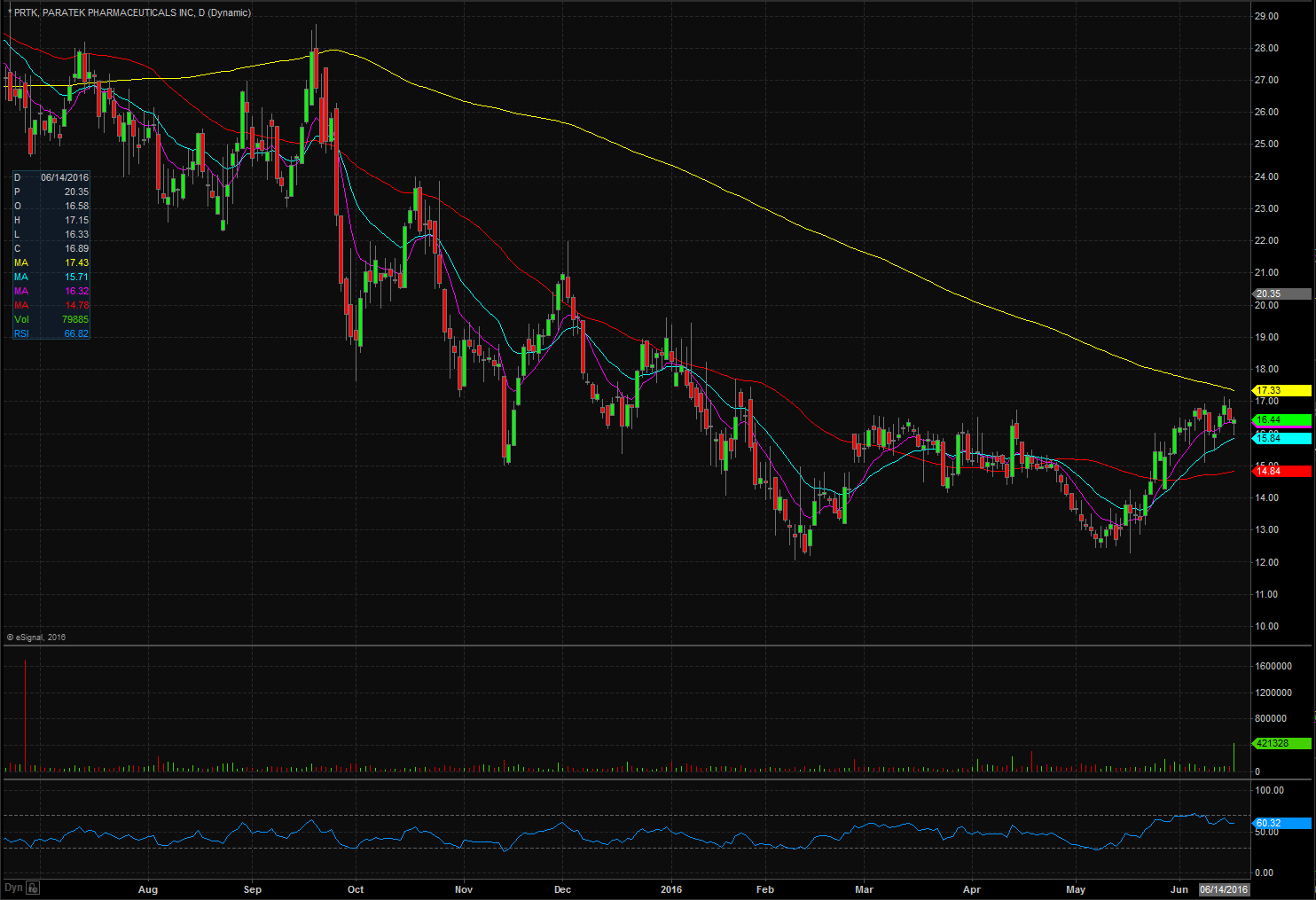
PRTK opened trading yesterday at $16.31 which was down from the previous days trading of $16.42. PRTK closed trading yesterday at $16.44 and spiked up after market to $20.30, equivalent to a 23% increase from the closing price. Taking a look at the daily chart we can see the last time PRTK traded above these levels was on December 2nd when it traded at $20.22 . Taking a closer look at the daily chart we can see that PRTK has been on a upward trend dating back to May 12th when it traded at $12.68. PRTK has a float of 10.92 million shares and traded below the normal daily trading volume on Thursday. For trading purposes, I would like to see QLGC open trading on Friday above $19.00 and if it does I would be looking to take a long position at the bell. My stop loss would be $0.20 from my entry position fearing anything more than that and the stock would start to fill in the gap up.
Company Profile
Paratek Pharmaceuticals, Inc., a clinical stage biopharmaceutical company, focuses on the development and commercialization of therapeutics based upon tetracycline chemistry in the United States. Its lead product candidates include Omadacycline, a broad-spectrum, intravenous, and oral antibiotic, which is in Phase III clinical for use as a monotherapy antibiotic for acute bacterial skin and skin structure infections (ABSSSI), community-acquired bacterial pneumonia (CABP), urinary tract infections, and other serious community-acquired bacterial infections; and Sarecycline, a tetracycline-derived compound that is in Phase III clinical trials for the treatment of acne and rosacea.
The company has special protocol assessment agreements with Food and Drug Administration for the Phase III trials planned in ABSSSI and CABP. It has collaborative research and license agreement with Allergan (NYSE:AGN_pa) plc to research, develop, and commercialize tetracycline products; license agreement with Tufts University to develop and commercialize products for the treatment or prevention of bacterial or microbial diseases, or medical conditions; and collaboration agreement with Purdue Pharmaceuticals L.P. to commercialize Intermezzo. Paratek Pharmaceuticals, Inc. is headquartered in Boston, Massachusetts.