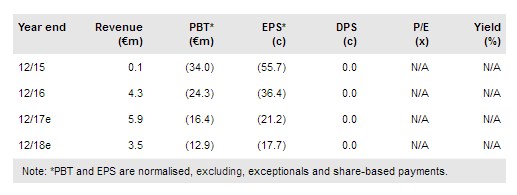
Paion O.N (DE:PA8G) is well positioned to commercialise Remimazolam in 2019 for procedural sedation (via partner COSMO Pharmaceuticals SA (SIX:COPN) in the US) and general anaesthesia (Japan); successful Phase III studies confirmed an excellent safety profile and shorter procedure times than midazolam. Paion is conducting additional Phase I studies to further assess abuse potential of remimazolam, which will guide its scheduling under the Controlled Substances Act in the US. Cosmo has advised that it expects to file for US approval in H218 vs prior guidance of a mid-2018 filing. The recent €8m capital raise has extended the funding runway for current activities to at least H219. We adjust our valuation to €245m (vs €240m) or €4.02/share.
Abuse studies ongoing, US filing expected H218
Paion is undertaking additional Phase I studies to assess whether remimazolam can be abused intranasally and whether it could be used as a knock-out cocktail in combination with alcohol. Paion expects that the FDA will classify remimazolam as a lower-abuse-potential schedule IV drug under the Controlled Substance Act, the same classification as midazolam. Paion has clarified the expected timeline for a potential US filing by partner Cosmo, with filing expected in H218 vs prior guidance of mid-2018. Encouragingly, Canadian regulators have advised partner Pharmascience that the current data package is adequate for filing in that country, without any further data from the current abuse potential trials.
To read the entire report Please click on the pdf File Below: