On 28 September 2019, Oryzon (MC:ORY) presented additional data from its Phase IIa CLEPSIDRA trial with iadademstat, a selective LSD1 inhibitor, at the ESMO congress in Barcelona. A poster presentation detailed the efficacy results from eight relapsed, extensive disease small cell lung cancer (ED SCLC) patients. In CLEPSIDRA patients receive 4–6 cycles of iadademstat plus carboplatin-etoposide chemotherapy (subsequently, the patients may be given iadademstat monotherapy). An objective response was seen in 6 out of 8 patients (75%). Of these, four patients demonstrated a partial response and two had long-term stable disease. Patients in the trial are stratified by proprietary biomarkers, which allow identifying SCLC sensitive to LSD1 inhibitors and can position iadademstat as a personalised therapy.
The objective response rate (ORR) of 75% compares well with the historical average of SCLC second-line chemotherapy drug topotecan (15–24%). SCLC is generally considered a non-immunogenic cancer, so objective response rates to immune checkpoint inhibitors are also relatively low (22% nivolumab plus ipilimumab; 19% pembrolizumab as monotherapy; Saleh, 2019). The high ORR in CLEPSIDRA may be due to the use of biomarkers. One of the patients with partial response showed 79% tumour reduction following six cycles of iadademstat plus carboplatin-etoposide and then received iadademstat as monotherapy. This patient is still in remission nine months after the treatment and demonstrated continuous improvement (86% tumour reduction) with iadademstat monotherapy.
The most common side effects were haematological changes seen in patients who received triple combination and included decreased platelets, neutrophils and anaemia. No other organ-specific side effects, such as neurological, hepatic or renal toxicity, were observed. In addition, iadademstat alone did not cause haematological or other toxicity. Such results are not unexpected as platinum-etoposide chemotherapy is known to have haematological toxicity. The fact that side effects were observed in patients receiving the combination treatment, but not iadademstat alone, would imply better tolerance of the latter. Oryzon will continue to explore different dosing regimens during the reminder of the CLEPSIDRA trial.
The rationale to test iadademstat for SCLC comes from the fact that the inhibition of LSD1 activates the NOTCH pathway resulting in the suppression of ASCL1 (a known SCLC tumour driver). Complete and durable tumour regression was seen with iadademstat in in vivo PDX models (discussed in our previous report). Oryzon will present more clinical data with its second asset vafidemstat from the REIMAGINE trial at the CINP this week, which we will cover in our next report.
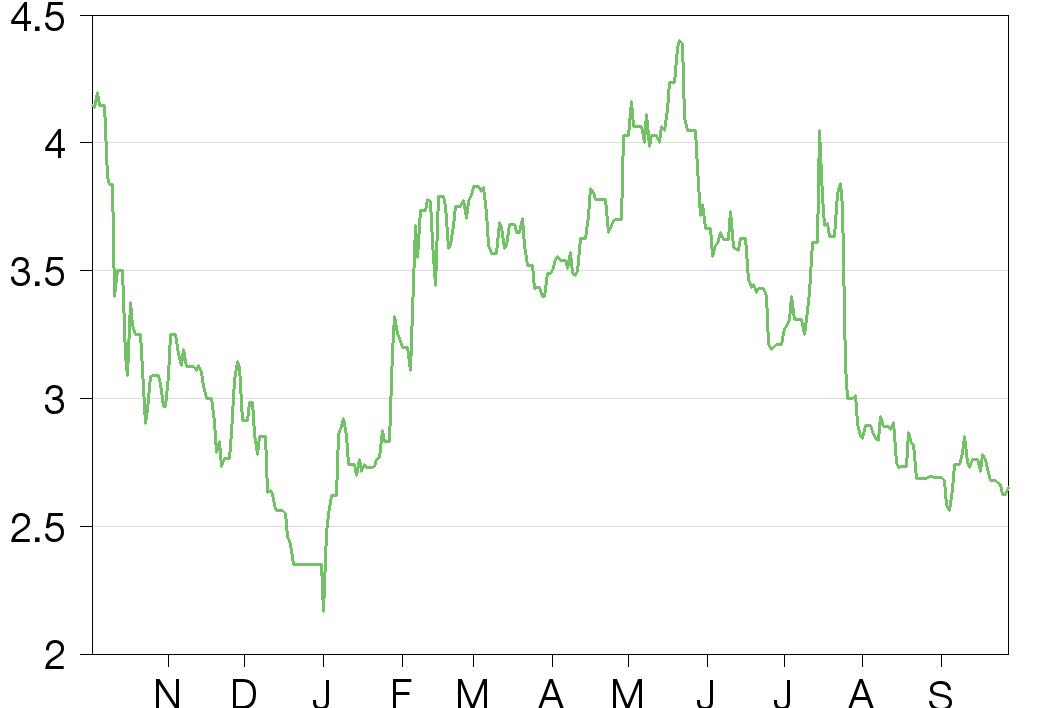
Share price performance
Business description
Oryzon Genomics is a Spanish biotech focused on epigenetics. Iadademstat (Phase IIa) is being explored for acute leukaemias and SCLC; vafidemstat, its CNS product, is in Phase IIa trials in MS, AD and aggression. Newer asset ORY-3001 is being developed for certain orphan indications.