Ophthotech Corporation (NASDAQ:OPHT) announced disappointing results from a phase III study —OPH1004 — evaluating its pipeline candidate Fovista (an anti-PDGF therapy) in combination with Regeneron Pharmaceuticals, Inc.’s (NASDAQ:REGN) Eylea (aflibercept) or Roche Holding (SIX:ROG) AG’s (OTC:RHHBY) Avastin (bevacizumab) for treatment of wet age-related macular degeneration (AMD).
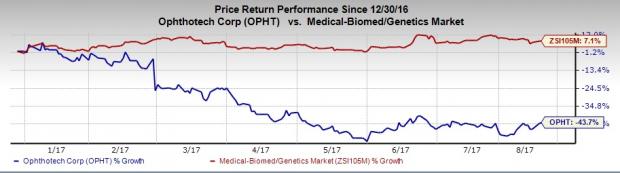
Ophthotech’s shares have significantly underperformed the industry so far this year. Shares of the company have lost 43.7%, comparing unfavorably with the industry’s increase of 7.1%.
The international, multi-center, randomized, double-masked, controlled phase III study evaluated the safety and efficacy of 1.5 mg of Fovista combined with Eylea or Avastin and compared with Eylea or Avastin monotherapy on patients with wet AMD. A total of 640 patients with AMD were enrolled in this trial.
Data revealed that the study failed to meet the pre-specified primary endpoint of a mean change in visual acuity at 12 months. Combined analysis from the two studies showed that patients who received Fovista combination therapy, gained a mean of 9.42 letters of vision on the Early Treatment of Diabetic Retinopathy Study (ETDRS) standardized chart at 12 months in comparison to a mean gain of 9.04 ETDRS letters for patients, who received Eylea or Avastin alone.
These results were not found to be statistically significant, thus leading to a conclusion that adding Fovista to a monthly Eylea or Avastin regimen did not result in any vision improvement in patients suffering from wet AMD. However, the Fovista combination therapy and Eylea or Avastin monotherapy were found to be well-tolerated after a year’s treatment, based on a preliminary analysis of the safety data.
The company believes, the poor results of the phase III study will not affect its strategy as it is moving ahead with multiple ongoing or planned clinical programs in orphan retinal diseases.
Notably, Ophthotech had signed a license and commercialization agreement with Novartis (NYSE:NVS) in May 2014. While Ophthotech holds the rights to Fovista in the U.S., Novartis owns the exclusive rights to the candidate outside the U.S.
We remind investors that in December 2016, the company announced disappointing results from two pivotal phase III studies — OPH1002 and OPH1003 — evaluating Fovista in combination with Novartis/Roche Holding’s Lucentis (an anti-VEGF therapy) for treating AMD.
With no approved product in Ophthotech’s portfolio and Fovista being the most advanced candidate in the company’s pipeline, the latest development will surely prove to be a huge blow to the company. Plus, the deal with Novartis is now shrouded in uncertainty.
Zacks Rank
Ophthotech currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Hottest Tech Mega-Trend of All
Last year, it generated $8 billion in global revenues. By 2020, it's predicted to blast through the roof to $47 billion. Famed investor Mark Cuban says it will produce "the world's first trillionaires," but that should still leave plenty of money for regular investors who make the right trades early.
See Zacks' 3 Best Stocks to Play This Trend >>
Roche Holding AG (RHHBY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
Ophthotech Corporation (OPHT): Free Stock Analysis Report
Original post
Zacks Investment Research