Shares of Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN) have moved up 14.9% over the last 30 days.
In fact, so far this year, Regeneron’s shares have rallied 43.4%, compared with the 12% gain of the Zacks classified Medical-Biomedical and Genetics industry.
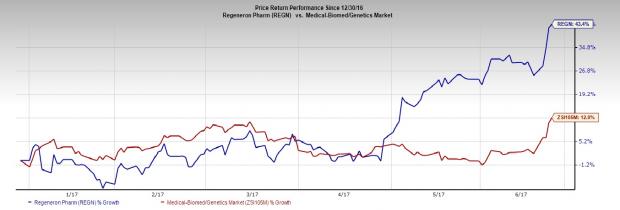
Let’s find out what is driving the stock.
Eylea continues to Drive Revenues
Regeneron’s key growth driver, Eylea, continues to drive revenues and the company is expanding the drug's label for additional indications.
Eylea is approved for the treatment of neovascular age-related macular degeneration (wet AMD) and diabetic macular edema (DME). The drug is also approved for macular edema following retinal vein occlusion, which includes macular edema post central retinal vein occlusion as well as macular edema following branch retinal vein occlusion.
Meanwhile, Regeneron is working on expanding Eylea label into additional indications for the treatment of moderately severe to severe non-proliferative diabetic retinopathy in patients without DME and neovascular glaucoma. Label expansion into additional indications would give Eylea access to higher patient population and increase the commercial potential of the drug.The company also received a boost when the FDA approved Dupixent (dupilumab) Injection for the treatment of adults with moderate-to-severe atopic dermatitis (AD). Pricing will play a key role in the uptake of the drug. Hence, we expect investors to focus on the performance of Dupixent and Kevzara. Both the drugs were developed in partnership with Sanofi (PA:SASY) (SNY).
Earlier in the week, Novartis (NYSE:NVS) released positive data on its wetAMD candidate, RTH258 (brolucizumab) which is poised to pose competition to Eylea. The candidate met the primary and key secondary endpoints in two phase III studies, HAWK and HARRIER-non-inferiority of RTH258 to Eylea in mean change in best-corrected visual acuity (BCVA) from baseline to week 48, and average mean change over the period of week 36-48, respectively. However, Novartis expects to complete the pharmacokinetic study with the final manufacturing process to enable filing in 2018 only. Hence, the candidate is not likely to hit markets before 2019 giving Eylea a breather.
Dupixent and Kevzara in Focus
Investors are upbeat about two key approvals so far in 2017 and looking forward to potential launches of these drugs.
In May the FDA approved Kevzara for treatment of adult patients with moderate to severe active rheumatoid arthritis who have an inadequate response to or intolerance to one or more biologic or non-biologic Disease-Modifying Anti-Rheumatic Drugs.
The company also received a boost when the FDA approved Dupixent (dupilumab) Injection for the treatment of adults with moderate-to-severe atopic dermatitis (AD). Pricing will play a key role in the uptake of the drug. Hence, we expect investors to focus on the performance of Dupixent and Kevzara. Both the drugs were developed in partnership with Sanofi (NYSE:SNY) .
Zacks Rank
Currently, Regeneron Pharma sports a Zacks Rank #1 (Strong Buy). Another top-ranked stock in healthcare sector is VIVUS, Inc. (NASDAQ:VVUS) with the same rank as Regeneron. You can see the complete list of today’s Zacks #1 Rank stocks here.
VIVUS’s loss per share estimates lessened from 50 cents to 39 cents for 2017 in the last 60 days. The company posted positive earnings surprises in all four trailing quarters with average beat of 233.69%
The Best & Worst of Zacks
Today you are invited to download the full, up-to-the-minute list of 220 Zacks Rank #1 "Strong Buys" free of charge. From 1988 through 2015 this list has averaged a stellar gain of +25% per year. Plus, you may download 220 Zacks Rank #5 "Strong Sells." Even though this list holds many stocks that seem to be solid, it has historically performed 6X worse than the market. See these critical buys and sells free >>
Sanofi (SNY): Free Stock Analysis Report
Novartis AG (NVS): Free Stock Analysis Report
Regeneron Pharmaceuticals, Inc. (REGN): Free Stock Analysis Report
VIVUS, Inc. (VVUS): Free Stock Analysis Report
Original post
