Ocular Therapeutix Inc. (NASDAQ:OCUL)
On Monday morning before the market opened, Ocular Therapeutic announced that its second Phase-III trial for its treatment of chronic allergic conjunctivitis failed to reach its primary endpoint, which sent shares tanking in the pre-market session. The treatment, Dextenza Intracanalicular Depot, was tested for treating the itching associated with chronic pink eye but the endpoint was determined between the difference in mean scores between a group receiving the treatment and another group receiving a placebo. Analysts currently have an average price target of $38.75 but that will most likely come down over the next few days.
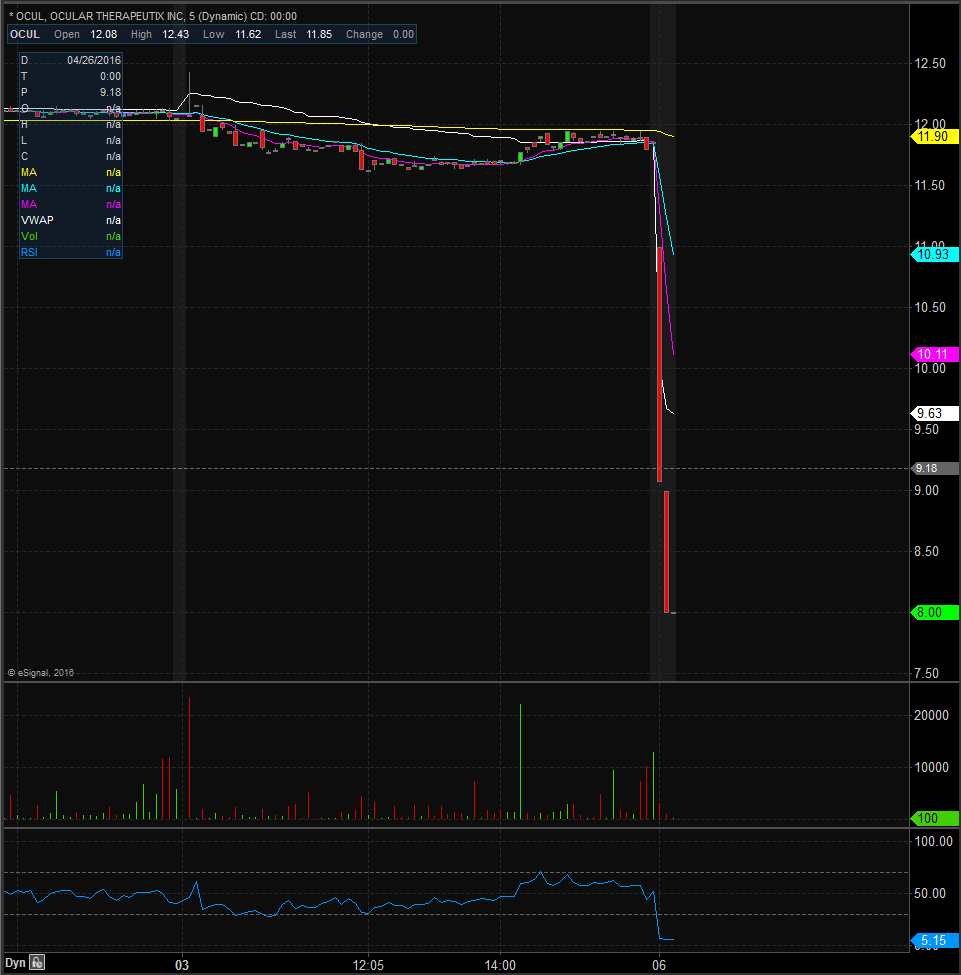
Looking at the chart you will notice that shares have been annihilated in the premarket session with current lows coming in at $8 after closing Friday at $11.85, representing a 32% drop in value. Shares had worked hard the past couple months to retake the 200-day moving average that is currently at $10.96 but now they will be well below it. We should see some support at $8, however, followed by $7.5 with resistance coming in at $9 and $10 and $10.96. There is also some ascending resistance just above the $10 mark so keep an eye on that level too. Look for shares to more volatile than normal as traders will be looking to take advantage of this big move.
CEO Comments
We are disappointed that the primary endpoint of ocular itching associated with allergic conjunctivitis was not achieved in our second Phase 3 trial in these patients, and this result is inconsistent with what we saw in our first Phase 3 trial,” said Amar Sawhney, Ph.D., President, Chief Executive Officer and Chairman. “We are currently in the process of conducting a thorough analysis of the data from the second Phase 3 trial to fully understand the difference in efficacy between the two Phase 3 trials. There was a greater variability in ocular itching exhibited by patients in the second Phase 3 trial over the multiple allergen challenges 7, 14 and 28 days following insertion of the depots, compared to the first Phase 3 trial. In a post hoc analysis, when ocular itching scores were averaged over these multiple visits, a statistically significant reduction of symptoms over the entire 1 month intended duration of sustained release, single dose therapy was observed in the DEXTENZA treatment group relative to the placebo vehicle group. Since this analysis was not part of the original end points, we plan to meet with the FDA to discuss the results and chart an appropriate path forward for the development of DEXTENZA for the treatment of allergic conjunctivitis.
Dr. Sawhney continued, We remain confident in the potential of our innovative sustained release platform to address diverse applications in ophthalmology. We look forward to the July 2016 PDUFA date for DEXTENZA for the treatment of post-surgical ocular pain.
OCUL Profile
Ocular Therapeutix, Inc., a biopharmaceutical company, focuses on the development and commercialization of therapies for diseases and conditions of the eye using its proprietary hydrogel platform technology in the United States. It markets ReSure Sealant, a hydrogel-based ophthalmic wound sealant to seal corneal incisions following cataract surgery. The company’s lead product include DEXTENZA, which is in Phase III clinical trial for the treatment of post-surgical ocular inflammation and pain during cataract surgery, as well as for the treatment of allergic conjunctivitis; and in Phase II clinical trial for the treatment of inflammatory dry eye disease. It is also developing OTX-TP, which has completed Phase IIb clinical trial for glaucoma and ocular hypertension. In addition, the company has a pipeline of earlier stage punctum plug product candidates, including OTX-MP, which has completed a Phase I clinical trial evaluating safety and pharmacokinetics in patients during cataract surgery; and evaluates sustained-release injectable anti-VEGF drug depots for back-of-the-eye diseases. Ocular Therapeutix, Inc. was founded in 2006 and is headquartered in Bedford, Massachusetts.