NuVasive Inc (NASDAQ:NUVA)
NuVasive, Inc. (NUVA), a medical device company yesterday reported their second quarter 2017 financial results. NUVA reported adjusted second quarter earnings of $0.46 per share which beat analyst expectations of $0.44 per share.
Second quarter revenues came in at $260.6 million which fell short of analyst expectations of $262 million.
NuVasive also announced the departure of their COO and CFO. COO John Hannon was stepping down to pursue other interests and CFO Quentin Blackford resigned.
NuVasive, Inc. CEO’s Comments
“NuVasive delivered better than expected operating profitability and earnings per share results in the second quarter 2017, along with continued strength across our International business, growing at more than 20% for the third quarter in a row,” said Gregory T. Lucier, chairman and chief executive officer of NuVasive. “In addition, several of our industry-disrupting technologies completed alpha and beta testing this quarter and will commercially launch over the next few months, giving surgeons and patients access to some of the most innovative technologies to address spine and trauma conditions, as well as radiation reduction in the operating room.”
NUVA Technical Analysis
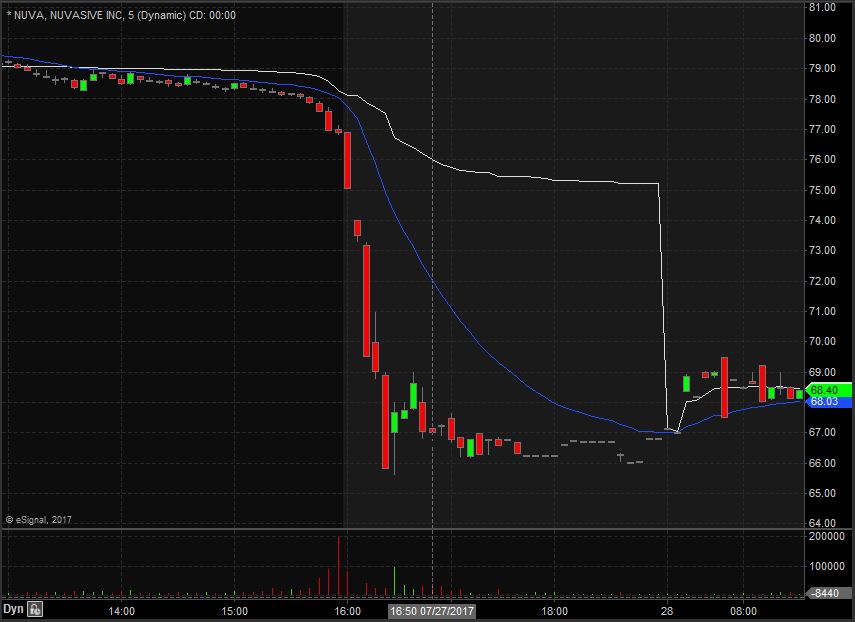
NUVA opened trading yesterday at $79.64 which was up from the previous day’s trading close of $79.46. Shares closed trading yesterday at $76.90 and spiked down after market to $66.80, equivalent to a 13% decrease from the closing price. Taking a look at the daily chart we can see the last time NUVA traded below these levels we have to go back to January 5th when it traded at lows of $66.77.
Taking a closer look at the daily chart we can see that before the spike down NUVA had been in an overall upward trend dating back to May 18th when it traded at $70.44. NUVA has a float of 50.27 million shares and traded 2.80 times the normal daily trading volume on Thursday.
For day trading purposes, I would like to see NUVA open trading on Friday below $70.00 and if it does I would be looking to take a short position at the bell. My stop loss would be $0.40 from my entry position fearing anything more than that and the stock would start to fill in the gap down.
Company Profile
NuVasive, Inc., a medical device company, develops and markets minimally-disruptive surgical products and procedurally-integrated solutions for spine surgery. Its products focus on applications for spine fusion surgery, including biologics used for spinal fusion process.
The company’s principal product is Maximum Access Surgery, a minimally-disruptive surgical platform, which includes its software-driven nerve detection and avoidance systems, NVM5, and intraoperative monitoring (IOM) services and support; MaXcess, an integrated split-blade retractor system; and various specialized implants and biologics.
Its spine surgery product line offerings comprise products for the thoracolumbar and the cervical spine, which are primarily used to enable surgeons access to the spine and to perform restorative and fusion procedures in a minimally-disruptive fashion.
Its biologics products include Osteocel Plus and Pro, a cellular bone matrix; Formagraft, a collagen-based synthetic bone substitute; AttraX, a synthetic bone graft material; and Propel DBM, a moldable demineralized bone matrix putty, which are used for spinal fusion or bone healing process. The company’s IOM services are used for onsite and remote monitoring of the neurological systems of patients undergoing spinal and brain-related surgeries.
It also provides implants; and fixation products, including pedicle screws, rods, and plates. In addition, the company offers Integrated Global Alignment platform for assessing, preserving, and restoring spinal alignment; MAGEC-early onset scoliosis, a spinal bracing and distraction system; and PRECICE, a limb lengthening system. NuVasive, Inc. sells its products to hospitals, surgeons, and other customers through independent sales agents, directly-employed sales personnel, and distributors in the United States and internationally. The company was founded in 1997 and is headquartered in San Diego, California.
