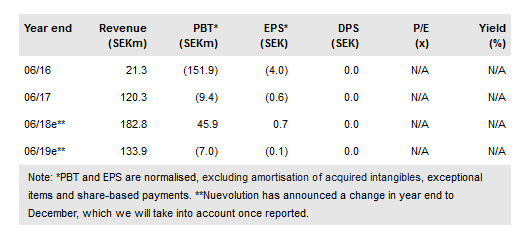
July to September 2017 results highlight the continued progression of Nuevolution AB (ST:NUEVOL) into a clinical company. Ongoing partnerships with Almirall, Amgen (NASDAQ:AMGN) and Janssen continue to advance as we expect and we anticipate that Almirall’s RORγt inhibitor programme will be the first Nuevolution asset to enter the clinic (in dermatology and psoriatic arthritis), which we forecast for H218/H119. Lead assets in the internal pipeline are nearing clinical readiness and Nuevolution anticipates a new partnership in the next three to 12 months. In the near term, up-listing to Nasdaq Stockholm’s Main Market and expansion of the investor base will position the company for future development. We value Nuevolution at SEK917m.
Collaborations continue to provide validation
Nuevolution’s core collaborations continue to progress as expected. In vivo proof of concept is expected in two cancer programmes in the Amgen Inc. (NASDAQ:AMGN) collaboration within the next three to six months. In the Almirall collaboration we expect Nuevolution to give a detailed update on the programme in H118; we forecast that it will enter the clinic in H218/H119. Nuevolution’s unpartnered assets are progressing well to clinical readiness, the RORγt inhibitor (outside Almirall-licensed indications) is in ongoing API production and the company expects to select the optimal indication for clinical development by year end. The BET BD1 programme is following closely behind with API production expected to start in H118. Nuevolution expects to announce a new collaboration/licensing deal in the next 3-12 months.
To read the entire report Please click on the pdf File Below: