Novo Nordisk (NYSE:NVO) has declared that its GLP-1 drugs for chronic obesity, under the brands Ozempic and Wegovy, are no longer in short supply. This caused the United States Food and Drug Administration (FDA) to take semaglutide, the active ingredient in both, off the drug shortage list on February 21, 2025.
This announcement sent shockwaves through shares of Hims Hers Health Inc (NYSE:HIMS), triggering an immediate 25% sell-off on the news from $59.60 to $49.28 and continued to sink another 33% to $32.90 in the following days. Novo Nordisk fell 10% at the market open on March 10.
The oligopoly of the GLP-1 industry, including maker Eli Lilly (NYSE:LLY), maker of tirzepatide name brand drugs Mounjaro and Zepbound, has once again sealed its dominance in the medical sector. However, Novo Nordisk took it one step further to crush its competitors and hammer another nail in the coffin of compounders when it slashed prices by more than 50%.
Dealing a Death Blow to Compounders
When semaglutide and tirzepatide supplies ran short, the drugs were put on the FDA drug shortage list. This special situation under 503B allowed compounding labs and facilities to manufacture compounded versions of the popular weight loss drugs without having to go through the rigorous FDA approval process. These treatments aren’t the same as generics, which are virtual duplicates of the original drugs but compounded versions, which may only have the active ingredient but not the exact same formula.
Many see this as a potentially dangerous loophole with no FDA regulatory approval process involved.
However, it’s been extremely lucrative for compounding labs and sellers of compounded versions like Him & Hers, which saw membership grow 45% YoY to 2.2 million and revenue surge 69% YoY to $1.5 billion in 2024. Their version of semaglutide sold for less than 70% of the list price (IE, Wegovy lists for $1,349.02) for the authentic versions, around $200 to $400 per month, depending on the payment plan.
NovoCare Pharmacy for $499 Wegovy
Taking a page from Eli Lilly and their LillyDirect online pharmacy, Novo Nordisk launched NovoCare Pharmacy, its direct-to-consumer (DTC) online pharmacy, where patients can get Wegovy for $499 per month, paying cash without using insurance. This applies to patients with prescriptions without insurance or with commercial insurance without coverage.
This option opens the door for patients who want to try Wegovy but don’t have coverage. It also enables patients using compounded versions to switch to the authentic version after compounders have ceased production. Novo Nordisk's Total Wegovy sales were $8.2 billion. Wegovy was launched in June 2021.
While Lilly opened their online pharmacy first, they didn’t go as far as offering cash discounts like Novo Nordisk. This could also start a race to the bottom between the oligopolies. However, Zepbound is proven to be more effective for weight loss than Wegovy since it targets two hormones, GLP-1 and GIP. Total Zepbound sales were $4.93 billion in 2024 for Lilly. Zepbound had a later start launch in November 2023. Tirzepatide was taken off the drug shortage list in December 2024.
Incidentally, shares of Novo Nordisk sank 17% prior to the FDA drug shortage supply announcement on disappointing results of its experimental obesity drug CagriSema, which only showed a 22.7% weight loss after 68 weeks, missing the 25% target.
The Next Generation of GLP-1 Drugs is Targeting up to 30% Weight Loss
While Novo Nordisk hit paydirt first with the off-label use of Ozempic for weight loss, Lilly surpassed them when they released Zepbound for obesity versus Wegovy. Under separate clinical trials, Zepbound produced a 72-week median weight loss of 19 to 20%, whereas Wegovy demonstrated 14 to 15%. Novo Nordisk's own dual agonist experimental drug, CagriSema, demonstrated 22.7% weight loss, which was still under its 25% target.
CagriSema Falls Further in Late-Stage Trial, But There Is a Caveat
To make matters worse, on March 10, 2025, Novo Nordisk reported its CagriSema Phase 3 REDEFINE 2 CagriSema late-stage study revealed just 13.7% weight loss versus 3.1% placebo in overweight type 2 diabetes patients over a 68-week period, which was short of its 15% or higher expectations. Its current obesity drug, Wegovy, had a 15% median weight loss at the 68-week mark.
However, Novo pointed out that 61.9% of patients were using the highest dosage. If all patients were on the highest dosage, then the weight loss would have been 15.7%. This study used a flexible protocol, like the REDEFINE 1 study, which allows patients to modify their dosages during the trial. Patients in the REDEFINE 2 study also had diabetes and were 5 kg heavier than patients in the first study.
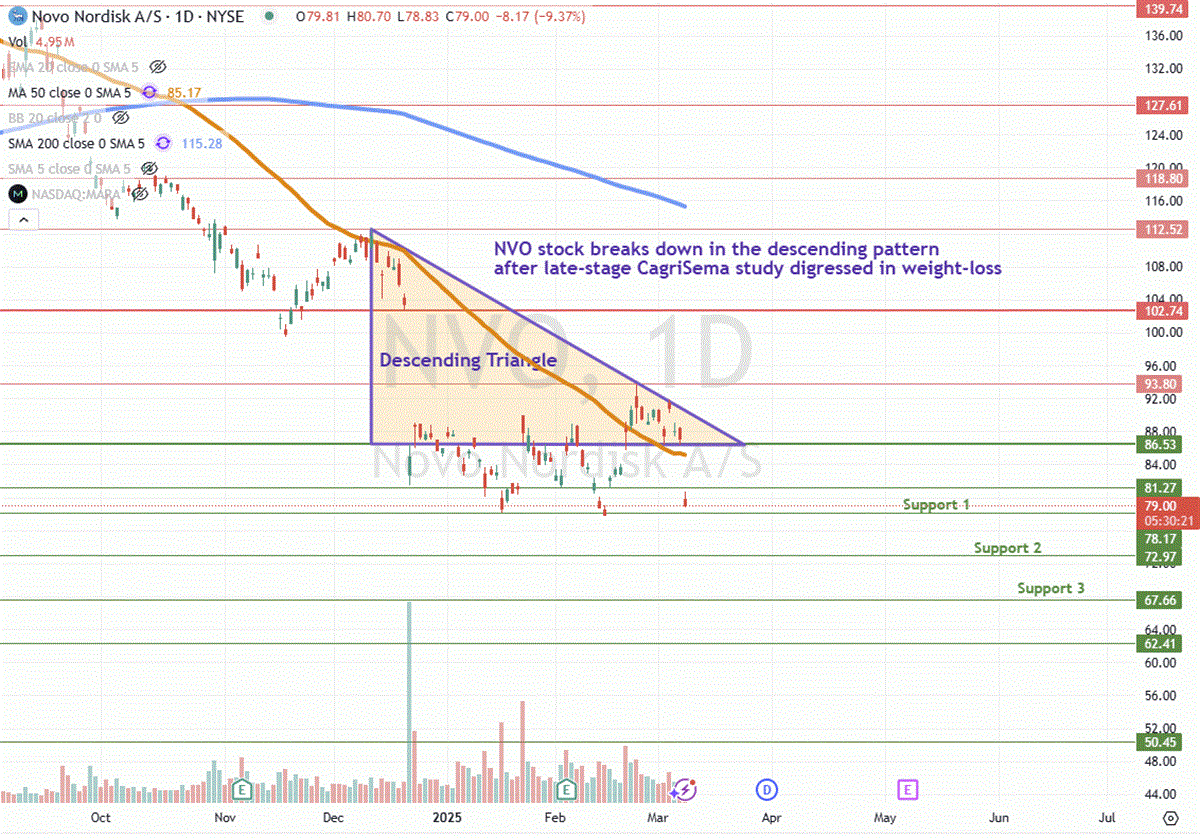
Nonetheless, the digression with the late-stage trial has caused sentiment to sour for the drug meeting its 25% weight loss goal, as demonstrated by the sell-off and breakdown.
Lilly’s Next-Generation Triple Agonist GLP-1 Drug Looks Very Promising
Lilly has also been working on their next-generation GLP-1 drug retratrutide, which is a triple agonist targeting GLP-1, GIP and glucagon. The 48-week Phase 2 study at the highest dose demonstrated the mean weight loss was 24.2% of body weight, which was completed in 2023. The Phase 3 study could result in 25 to 30% mean weight loss. While there is no exact date, there is speculation it may seek FDA approval in 2025.
