Healing sore eyes
NovaBay Pharmaceuticals Inc, (NBY) is facing a pivotal year in 2014. Results from a global 450-patient Phase IIb study of its lead anti-infective agent, auriclosene, in viral conjunctivitis are expected in mid-2014. Positive data could re-rate the stock and offer the potential to secure fresh finance and new partnerships. Further clinical studies are also expected to start in H214, for impetigo and urinary catheter irrigation. Auriclosene’s potential to overcome microbial resistance could play well with renewed regulatory and investor interest in the anti-infectives sector. We value NovaBay at $80m, or $1.75 per share.
An important read-out
The 450-patient viral conjunctivitis study is the biggest trial conducted so far with auriclosene. A previous Phase II study, conducted by former partner Alcon, missed the primary endpoint but was underpowered as it failed to recruit sufficient numbers of adenovirus positive patients. However, improvements in microbiological success and blurred vision were observed in patients with a more severe, sight-threatening sub-type called epidemic keratoconjunctivitis (EKC). The current study is using an FDA-approved point-of-care adenoviral screening test to ensure correct patient enrolment, and the company estimates that two-thirds of patients will have EKC
Further development expected
NovaBay plans to initiate further clinical studies with auriclosene in 2014. For urinary catheter blockage and encrustation (UCBE), highly encouraging Phase IIa data could enable a potentially pivotal Phase IIb/III study. In impetigo, a Phase IIb study conducted by partner Galderma missed its primary efficacy endpoint, but NovaBay intends to initiate another Phase IIb trial with an optimised formulation that had previously generated encouraging Phase IIa results. The size and scope of both studies still need to be agreed with the FDA, and discussions are underway.
Renewed interest in anti-infectives
A CDC report (Threat Report 2013) highlighted the urgent need for new therapies to combat bacterial resistance to conventional antibiotics, while the recently proposed ADAPT Act (to replace the GAIN Act) provides an expedited pathway to FDA approval for antibiotics/antifungals, particularly in limited and specific patient populations. These initiatives, and constant reports of the threat posed by antibiotic resistance, have certainly reinvigorated interest in the anti-infectives sector.
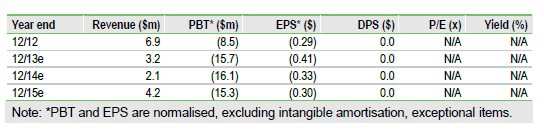
Valuation: $80m, $1.75 per share, ahead of data
We value NovaBay at $80m, or $1.75 per share, based on a sum-of-the-parts DCF model. This is driven by auriclosene’s potential in viral conjunctivitis ($250m peak sales) and UCBE ($75m) and is fair value for the stock ahead of catalysts in 2014.